BREAKING: FDA Pulls Valneva Chikungunya Vaccine From Market Over "Serious Safety Concerns"
Bio-Pharmaceutical Complex paves the way for Bavarian Nordic’s virus-like particle shot — while ignoring millions of deaths, injuries, and disabilities from COVID-19 genetic products.
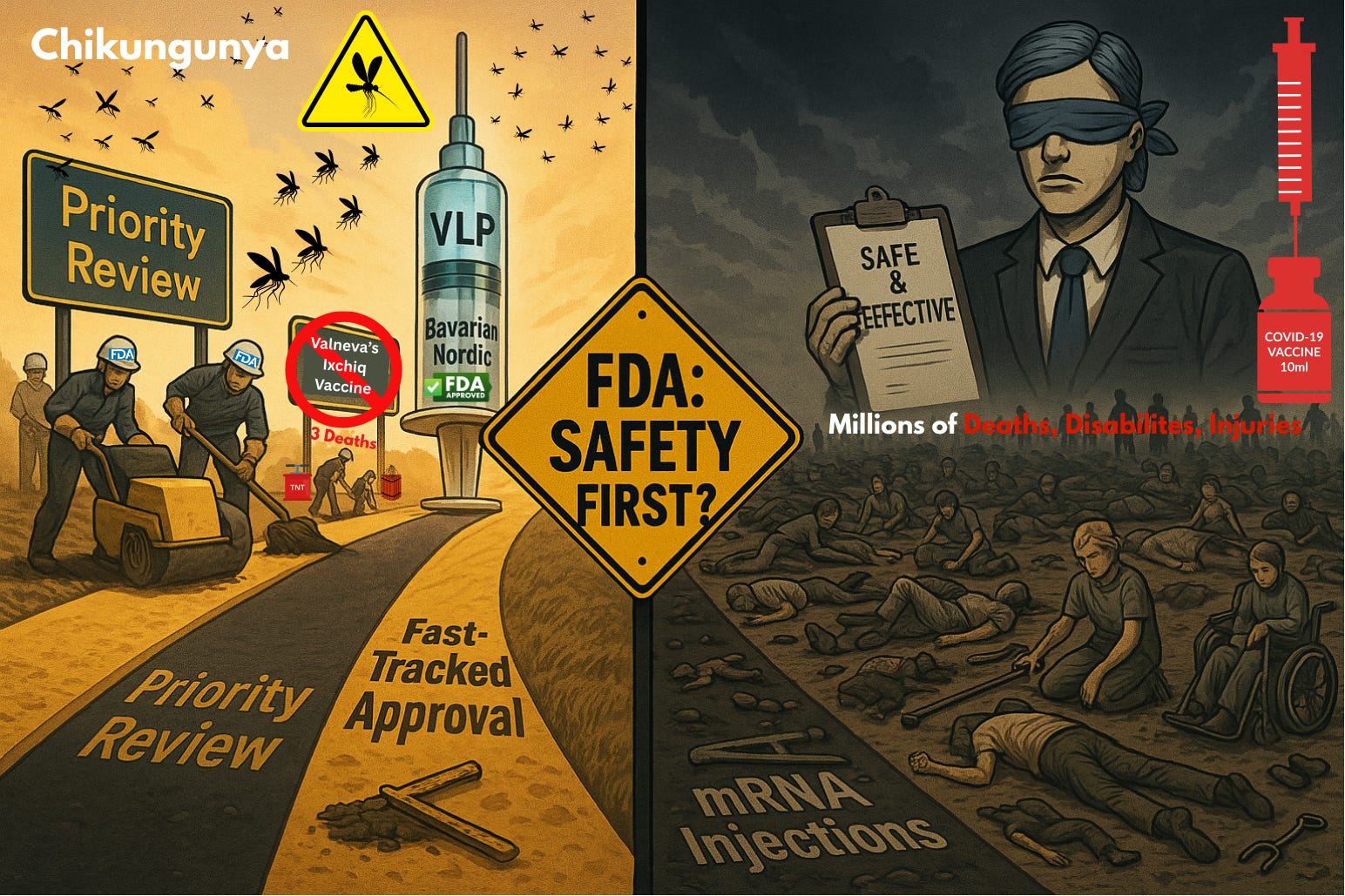
The FDA has just suspended approval of Valneva’s live-attenuated chikungunya vaccine Ixchiq, citing serious safety concerns—including 21 hospitalizations, 3 deaths, and confirmed cases of “chikungunya-like illness” triggered by the shot itself. The agency admitted the risks outweigh benefits “under most plausible scenarios.”
On the surface, this looks like overdue regulatory accountability. But step back—and the picture is far murkier.
The Convenient Timing
Just months earlier, in February 2025, the FDA approved Bavarian Nordic’s competing chikungunya vaccine, VIMKUNYA™, under “Priority Review.” Bavarian Nordic’s shot is approved for everyone 12 and up.
As part of the deal, Bavarian Nordic was also awarded a Tropical Disease Priority Review Voucher (PRV) — a golden ticket in the vaccine world that can be sold or used to fast-track future products, often worth hundreds of millions of dollars.
Now, the only chikungunya vaccine left standing in the U.S. is VIMKUNYA, a virus-like particle (VLP) vaccine. Here’s how it works according to Bavarian Nordic:
The Bio-Pharmaceutical Complex at Work
This is how the Bio-Pharmaceutical Complex operates:
Accelerated approvals rush risky products (Ixchiq) into circulation before benefits are confirmed.
Safety crises are inevitable—yet they provide a controlled opportunity to redirect market share.
Regulators and industry insiders decide which companies are protected and which are sacrificed.
Valneva takes the fall. Bavarian Nordic—aligned with regulators, armed with political capital, and flush with government-awarded vouchers—takes the win.
The Double Standard
Here’s the most glaring problem:
Ixchiq was pulled after 3 deaths and ~20 severe cases.
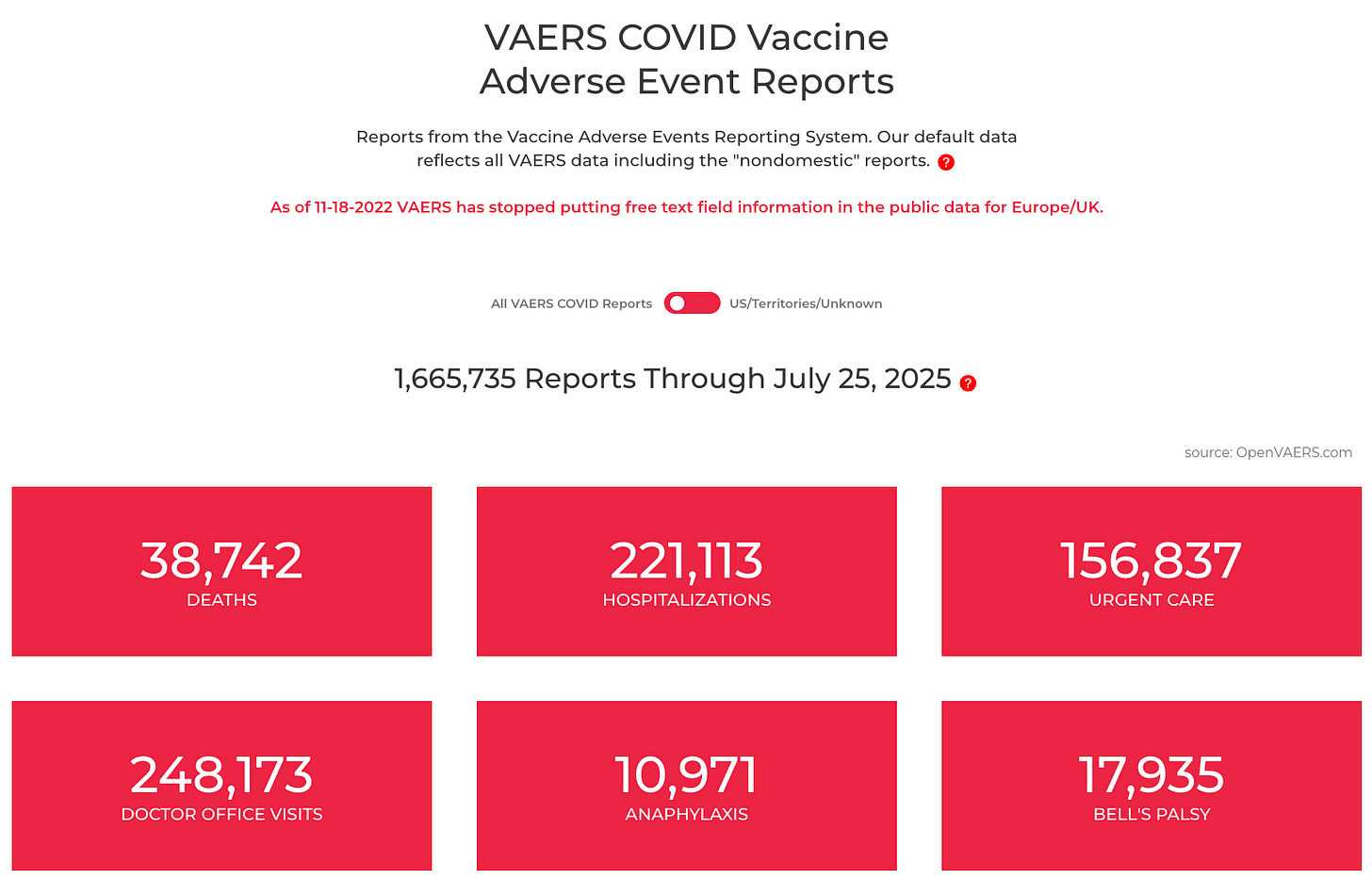
Meanwhile, the COVID-19 mRNA injections have been linked to millions of deaths, disabilities, and severe injuries.
Yet the FDA, CDC, and WHO have taken no such market-withdrawal action. Instead, these injections remain shielded by liability protections and continue to be pushed onto populations, including children and pregnant women.
The contrast is stunning: a minor player gets sacrificed to make way for the chosen giant, while the flagship mRNA products—despite orders of magnitude more harm—remain untouchable.
The FDA framed Ixchiq’s withdrawal as a matter of safety. But given the timing, it looks just as much like a market-shaping maneuver.
Once again, the public is the proving ground. Once again, the chosen winners profit. And once again, the injuries and deaths from the COVID-19 mRNA injections are ignored—because pulling those products would threaten the very foundation of the Bio-Pharmaceutical Complex itself.
Epidemiologist and Foundation Administrator, McCullough Foundation
Support our mission: mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.





Oh so now the FDA is concerned about safety...😆
Hey Nicholas! I am a former nurse practitioner who was on the front lines of Covid and the Denton Texas area. My Texas legislator is having me attend a medical freedom conference with him on September 6. I would love to contact you personally and see what you would like him to have in his hands.!