BREAKING: Trump Bans Foreign Gain-of-Function Research — U.S. Experiments Paused
The executive order halts federal funding for dangerous virus experiments overseas and temporarily suspends high-risk U.S. research involving infectious pathogens and toxins.
President Donald Trump signed an executive order today titled, IMPROVING THE SAFETY AND SECURITY OF BIOLOGICAL RESEARCH, halting all federal funding for dangerous gain-of-function research in China, Iran, and other countries lacking adequate oversight. The order also suspends federally funded research involving infectious pathogens and toxins within the United States until a new, enforceable oversight policy is developed.
By the authority vested in me as President by the Constitution and the laws of the United States of America, it is hereby ordered:
Section 1. Purpose. Dangerous gain-of-function research on biological agents and pathogens has the potential to significantly endanger the lives of American citizens. If left unrestricted, its effects can include widespread mortality, an impaired public health system, disrupted American livelihoods, and diminished economic and national security.
The Biden Administration allowed dangerous gain-of-function research within the United States with insufficient levels of oversight. It also actively approved, through the National Institutes of Health, Federal life-science research funding in China and other countries where there is limited United States oversight or reasonable expectation of biosafety enforcement.
This recklessness, if unaddressed, may lead to the proliferation of research on pathogens (and potential pathogens) in settings without adequate safeguards, even after COVID-19 revealed the risk of such practices.
Sec. 2. Policy. It is the policy of the United States to ensure that United States federally funded research benefits American citizens without jeopardizing our Nation’s security, strength, or prosperity. My Administration will balance the prevention of catastrophic consequences with maintaining readiness against biological threats and driving global leadership in biotechnology, biological countermeasures, biosecurity, and health research.
Sec. 3. Stop Dangerous Gain-of-Function Research. (a) The Director of the Office of Science and Technology Policy (OSTP), in coordination with the Director of the Office of Management and Budget and the Assistant to the President for National Security Affairs (APNSA), and in consultation with the Secretary of Health and Human Services and the heads of other relevant executive departments and agencies (agencies) identified by the Director of OSTP, shall establish guidance for the heads of relevant agencies, to the extent consistent with the terms and conditions of the funding, to immediately:
(i) end Federal funding of dangerous gain-of-function research conducted by foreign entities in countries of concern (e.g., China) pursuant to 42 U.S.C. 6627(c), or in other countries where there is not adequate oversight to ensure that the countries are compliant with United States oversight standards and policies; and
(ii) end Federal funding of other life-science research that is occurring in countries of concern or foreign countries where there is not adequate oversight to ensure that the countries are compliant with United States oversight standards and policies and that could reasonably pose a threat to public health, public safety, and economic or national security, as determined by the heads of relevant agencies.
(b) The Director of OSTP, in coordination with the Director of the Office of Management and Budget and the APNSA, and in consultation with the Secretary of Health and Human Services and the heads of other relevant agencies, shall establish guidance for the Secretary of Health and Human Services and the heads of other relevant agencies with respect to suspension of federally funded dangerous gain-of-function research, pursuant to the terms and conditions of the relevant research funding, at least until the completion of the policy called for in section 4(a) of this order. Heads of agencies shall report any exception to a suspension to the Director of OSTP for review in consultation with the APNSA and the heads of relevant agencies.
Sec. 4. Secure Future Research Through Commonsense Frameworks. (a) Within 120 days of the date of this order, the Director of OSTP, pursuant to 42 U.S.C. 6627 and in coordination with the APNSA and the heads of relevant agencies, shall revise or replace the 2024 “United States Government Policy for Oversight of Dual Use Research of Concern and Pathogens with Enhanced Pandemic Potential” to:
(i) strengthen top-down independent oversight; increase accountability through enforcement, audits, and improved public transparency; and clearly define the scope of covered research while ensuring the United States remains the global leader in biotechnology, biological countermeasures, and health research;
(ii) incorporate enforcement mechanisms, including those described in section 7 of this order, into Federal funding agreements to ensure compliance with all Federal policies governing dangerous gain-of-function research; and
(iii) provide for review and revision at least every 4 years, or as appropriate.
(b) Within 90 days of the date of this order, the Director of OSTP, in coordination with the APNSA and the heads of relevant agencies, shall revise or replace the 2024 “Framework for Nucleic Acid Synthesis Screening” (Framework) to ensure it takes a commonsense approach and effectively encourages providers of synthetic nucleic acid sequences to implement comprehensive, scalable, and verifiable synthetic nucleic acid procurement screening mechanisms to minimize the risk of misuse. The heads of all agencies that fund life-science research shall ensure that synthetic nucleic acid procurement is conducted through providers or manufacturers that adhere to the updated Framework. To ensure compliance, the updated Framework shall incorporate the enforcement mechanisms described in section 7 of this order. The Framework shall be reviewed and revised at least every 4 years, or as appropriate
Sec. 5. Manage Risks Associated with Non-federally Funded Research. Within 180 days of the date of this order, the Director of OSTP, in coordination with the Director of the Office of Management and Budget, the APNSA, the Assistant to the President for Domestic Policy, and the heads of other relevant agencies, shall develop and implement a strategy to govern, limit, and track dangerous gain-of-function research across the United States that occurs without Federal funding and other life-science research that could cause significant societal consequences. This strategy shall include actions to achieve comprehensive, scalable, and verifiable nucleic acid synthesis screening in non-federally funded settings. Any gaps in authorities necessary to achieve the goals of this strategy shall be addressed in a legislative proposal to be sent to the President, through the Director of OSTP and the APNSA, within 180 days of the date of this order.
Sec. 6. Increase Accountability and Public Transparency of Dangerous Gain-of-Function Research. The Director of OSTP, in coordination with the APNSA and the heads of relevant agencies, shall ensure that the revised policy called for in section 4(a) of this order includes a mechanism whereby research institutions that receive Federal funding must report dangerous gain-of-function research, and to the maximum extent permitted by law, include research that is supported by non-Federal funding mechanisms. The reporting mechanism shall provide a publicly available source of information about research programs and awards identified pursuant to this section, including, where permitted by law, those that have been stopped or suspended pursuant to sections 3(a) and 3(b) of this order, and all future programs and awards that are covered by the updated policy developed in section 4(a) of this order. This reporting shall be conducted in a way that does not compromise national security or legitimate intellectual property interests of subject institutions.
Sec. 7. Future Enforcement Terms. The Secretary of Health and Human Services and the heads of other relevant agencies shall, consistent with existing laws and regulations, include in every life-science research contract or grant award:
(a) a term requiring the contractual counterparty or grant recipient to agree that its compliance in all respects with the terms of this order and any applicable regulations promulgated by the contracting or grant-offering agency is material to the Government’s payment decisions for purposes of 31 U.S.C. 3729(b)(4);
(b) a term requiring such counterparty or recipient to certify that it does not operate, participate in, or fund any dangerous gain-of-function research or other life-science research in foreign countries that could cause significant societal consequences or generate unnecessary national security risks, and that does not comply with this order and the policies ordered herein;
(c) a term stating that a violation of the terms of this order or any applicable regulations promulgated by the contracting or grant-offering agency by any grant recipient may be considered a violation of such term by the recipient’s employer or institution; and
(d) a term stating that any grant recipient, employer, or institution found to be in violation of the terms of this order or any applicable regulations promulgated by the contracting or grant-making agency may be subject to immediate revocation of ongoing Federal funding, and up to a 5-year period of ineligibility for Federal life-sciences grant funds offered by the Department of Health and Human Services and other relevant agencies.
Sec. 8. Definitions. For the purposes of this order,
“dangerous gain-of-function research” means scientific research on an infectious agent or toxin with the potential to cause disease by enhancing its pathogenicity or increasing its transmissibility. Covered research activities are those that could result in significant societal consequences and that seek or achieve one or more of the following outcomes:
(a) enhancing the harmful consequences of the agent or toxin;
(b) disrupting beneficial immunological response or the effectiveness of an immunization against the agent or toxin;
(c) conferring to the agent or toxin resistance to clinically or agriculturally useful prophylactic or therapeutic interventions against that agent or toxin or facilitating their ability to evade detection methodologies;
(d) increasing the stability, transmissibility, or the ability to disseminate the agent or toxin;
(e) altering the host range or tropism of the agent or toxin;
(f) enhancing the susceptibility of a human host population to the agent or toxin; or
(g) generating or reconstituting an eradicated or extinct agent or toxin.
Sec. 9. General Provisions. (a) Nothing in this order shall be construed to impair or otherwise affect:
(i) the authority granted by law to an executive department or agency, or the head thereof; or
(ii) the functions of the Director of the Office of Management and Budget relating to budgetary, administrative, or legislative proposals.
(b) This order shall be implemented consistent with applicable law and subject to the availability of appropriations.
(c) This order is not intended to, and does not, create any right or benefit, substantive or procedural, enforceable at law or in equity by any party against the United States, its departments, agencies, or entities, its officers, employees, or agents, or any other person.
(d) The Department of Health and Human Services shall provide funding for this order’s publication in the Federal Register.
DONALD J. TRUMP
THE WHITE HOUSE,
May 5, 2025.
This executive order marks a critical first step toward ending dangerous gain-of-function research once and for all. It appears to pause ongoing experiments in the U.S. involving infectious pathogens and toxins — a welcome and long-overdue action to protect the American people.
Notably, the order also acknowledges a long-ignored danger: non-federally funded GOF research in private labs and universities. For the first time, the U.S. government is directing agencies to track and regulate high-risk bioengineering activities outside federal funding channels — a move that could close major loopholes exploited in past pandemic-related experiments.
However, we must remain clear-eyed — a temporary pause is not a permanent solution. Some of the most reckless and high-risk gain-of-function experiments have taken place right here on U.S. soil:
Chimeric Coronavirus Engineering at UNC-Chapel Hill
Ralph Baric and colleagues, funded by the NIH through Peter Daszak and the EcoHealth Alliance, published two papers back in 2015 and 2016 highlighting their creation of chimeric SARS-like viruses at the University of North Carolina at Chapel Hill and the Wuhan Institute of Virology:
A recent study identified seven laboratory-acquired infections (LAIs) with SARS-CoV-2, sequenced from June 2020 to January 2021 at the University of North Carolina (UNC). These infections are suspected to have originated from synthetic virus research conducted under BSL-3 conditions at premier UNC coronavirus laboratories, including the Baric Lab led by the infamous Dr. Ralph Baric:
H5N1 Bird Flu Gain-of-Function at the University of Wisconsin–Madison
The Gates Foundation gave $9.5 million to UW-Madison and principal investigator Yoshihiro Kawaoka to modify H5N1 viruses to preferentially recognize human-type receptors and transmit efficiently in mammals. The Gates Foundation money was also used in a project headed by both Yoshihiro Kawaoka and Ron Fouchier (he previously modified H5N1 to become airborne transmissible in ferrets at the Erasmus Medical Center), where they provided the two additional mutations that would be needed in Egyptian H5N1 viruses to create variants with the mammalian “transmissibility features” identified in the Kawaoka study:
H5N1 Bird Flu Serial Passage Gain-of-Function at the USDA Southeast Poultry Research Laboratory (SEPRL)
Our peer-reviewed study, Proximal Origin of Epidemic Highly Pathogenic Avian Influenza H5N1 Clade 2.3.4.4b and Spread by Migratory Waterfowl, found that the current H5N1 bird flu outbreak may have originated from the USDA Southeast Poultry Research Laboratory (SEPRL). This has not been challenged by any U.S. government agency:
Blacksell et al found that, since 2001, there have been 309 confirmed and reported lab-acquired infections globally, with a vast majority (78.6%) occurring in the United States.
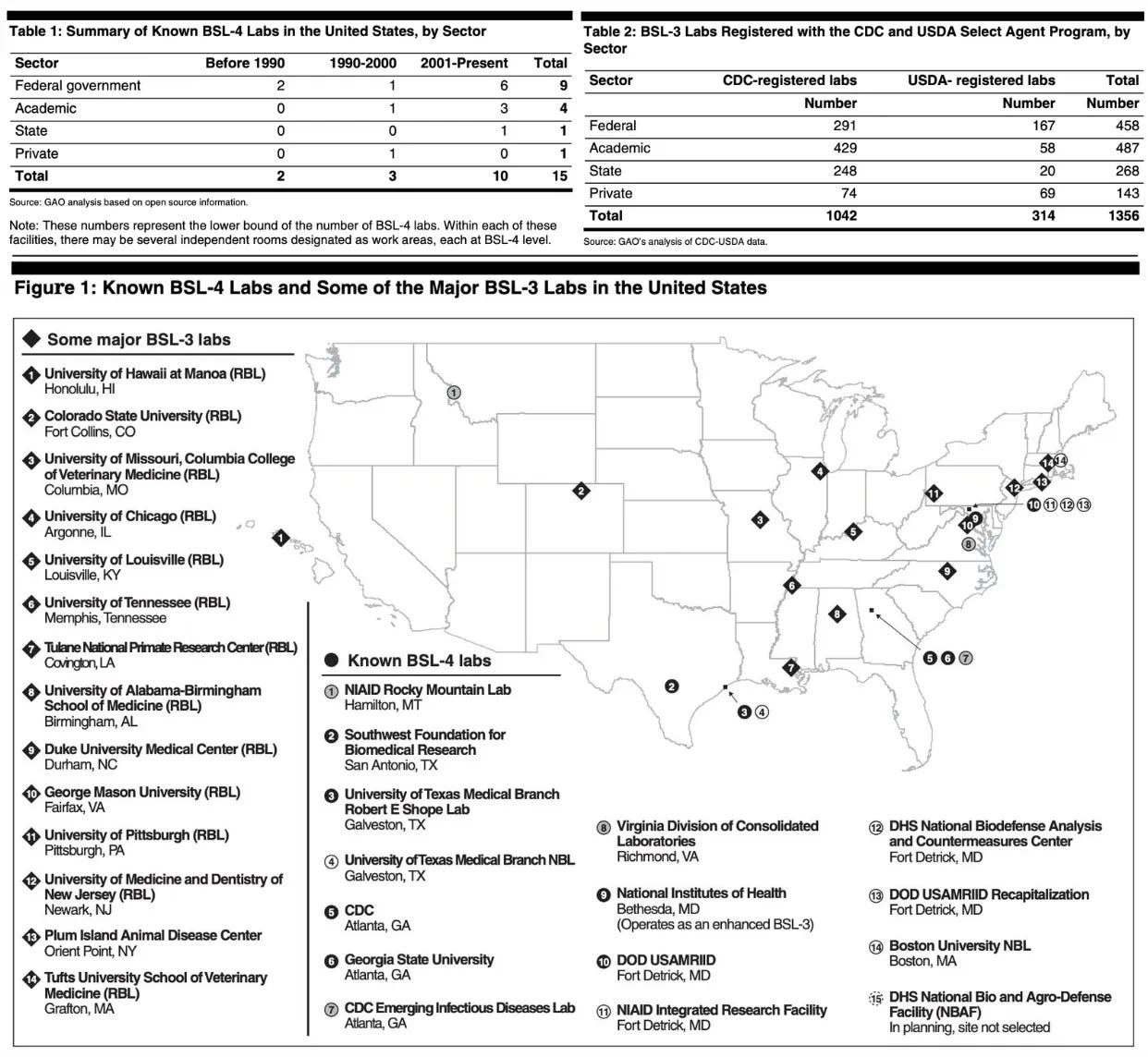
According to a United States Government Accountability Office (GAO) document, as of 2007, the U.S. had 1,356 BSL-3 biolabs and 15 BSL-4 biolabs—these numbers are almost certainly much higher today (exact figures unavailable):
These domestic programs pose just as much — if not more — of a threat to public health and national security as their foreign counterparts.
The new executive order is a critical step in the right direction — but without a permanent, enforceable ban on domestic and private gain-of-function research, the next pandemic could still be manmade.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.









Wasn’t Gain of Function already illegal? So, when Fauci & Co get caught, no charges? They just submit another bill banning it again?
President Trump and HHS Secretary Bobby Kennedy
Thank you for coming through for us. I knew you would. What a blessing to have you both working together and supporting each other.