Chikungunya Viral Outbreak in Asia
Estimated 35 Million Annual Cases, Common Mosquito-Borne RNA Virus Calls for Mosquito Control
By Peter A. McCullough, MD, MPH
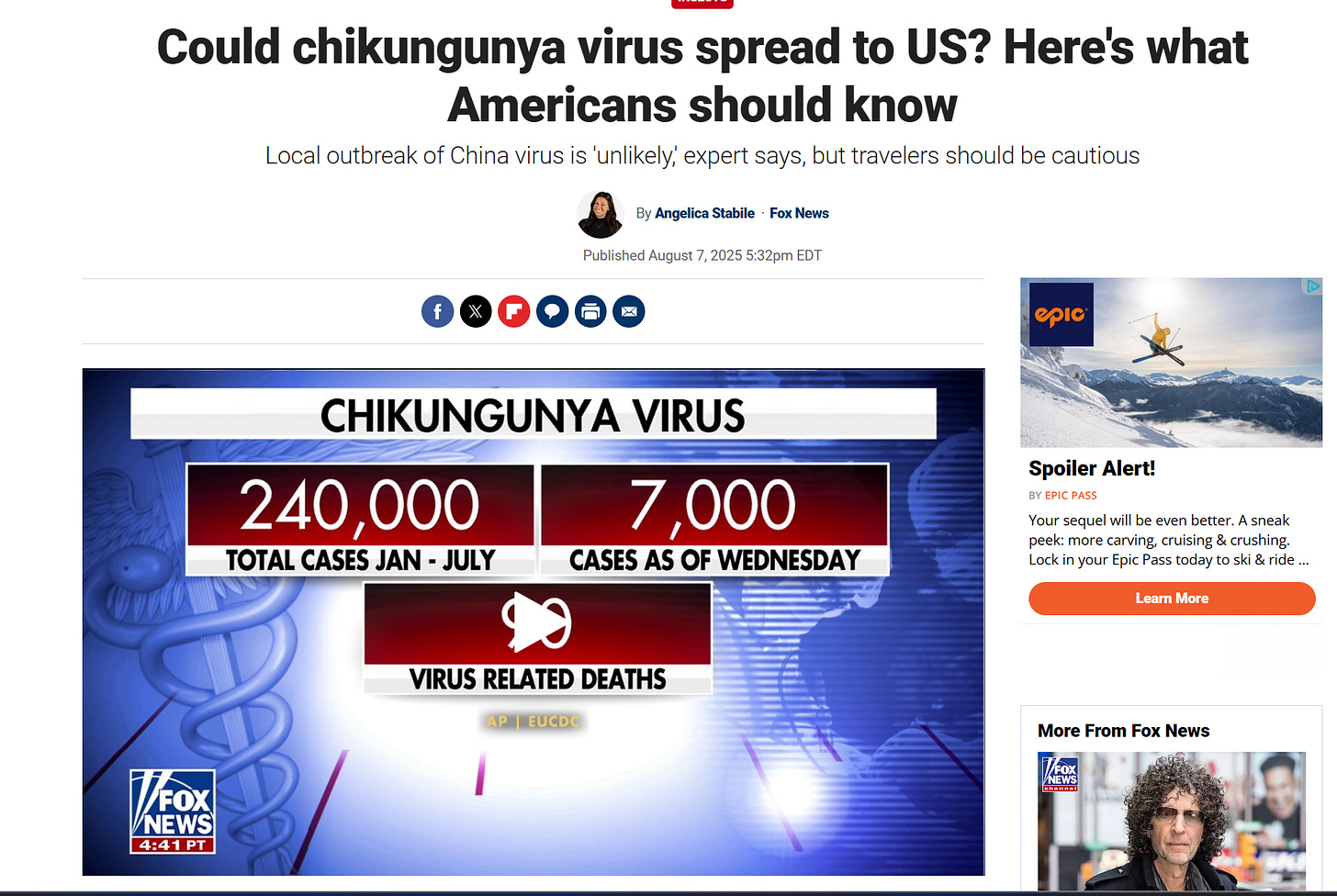
Chikungunya disease is a common RNA viral illness transmitted to humans through the bite of infected Aedes mosquitoes. Ribeiro Dos Santos et al published on June 10, 2025, estimates of approximately 35 million chikungunya virus infections annually worldwide. It is characterized by symptoms like fever and severe joint pain, which can be debilitating for some individuals. While most people recover within a week, some may experience long-lasting joint pain.
Yes, there is a chikungunya disease outbreak in China, specifically in Guangdong Province. The outbreak is concentrated in Foshan city, with thousands of confirmed infections. Officials are implementing aggressive containment measures, including public awareness campaigns and vector control efforts.
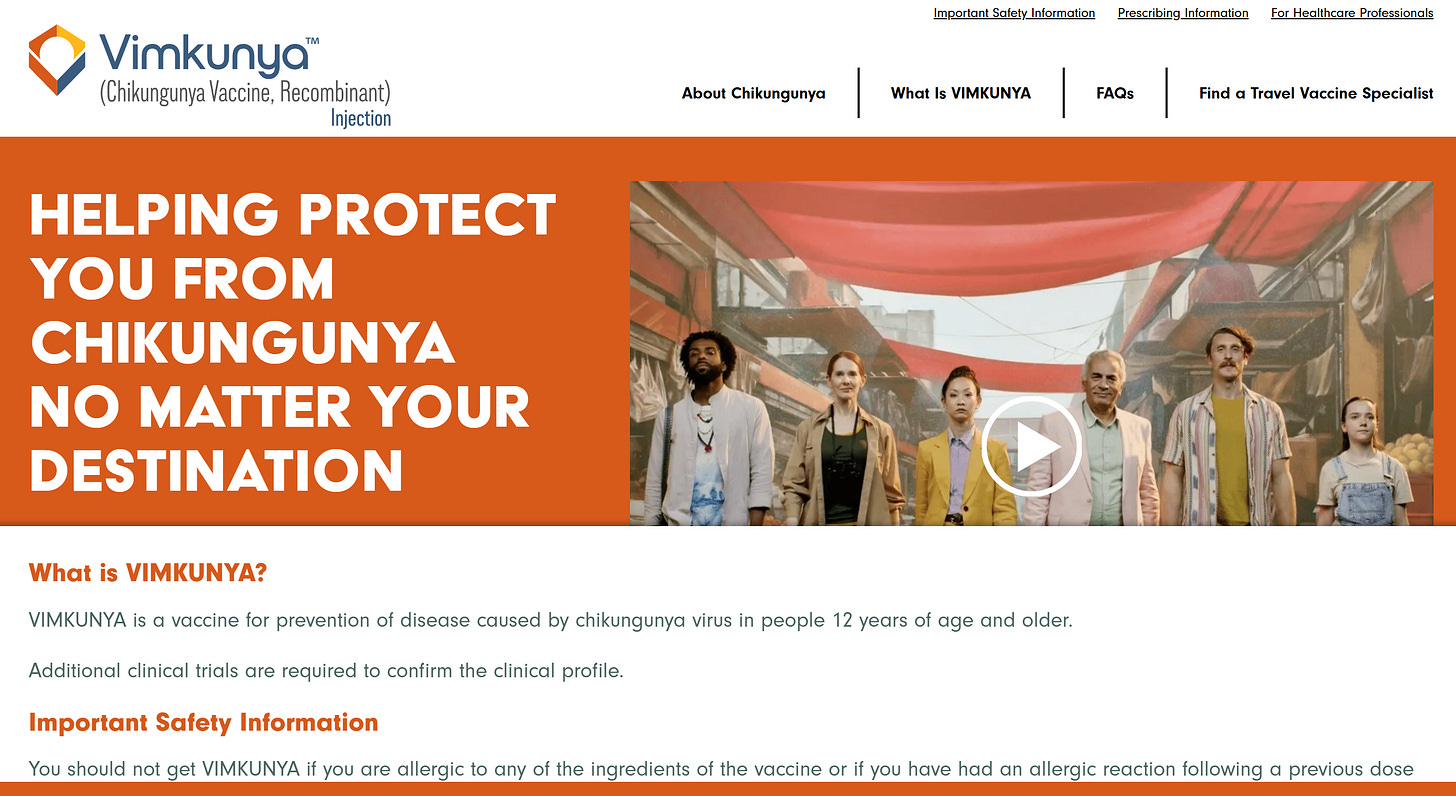
It should come as no surprise there two recently approved chikungunya vaccines available in the U.S.: a live-attenuated vaccine called IXCHIQ and a virus-like particle vaccine called VIMKUNYA.
The first chikungunya vaccine approved in the US, Ixchiq, was licensed by the Food and Drug Administration (FDA) in November 2023, based on antibody titers. It is a live-attenuated vaccine manufactured by Valneva and is approved for individuals 18 years and older at increased risk of chikungunya exposure. Study 1: A randomized, placebo-controlled, double-blinded study included 3,082 participants who received Ixchiq and 1,033 who received a placebo. Study 2: A non-placebo-controlled study where 408 participants were vaccinated with Ixchiq.
The second vaccine, Vimkunya, was approved in February 2025 for individuals 12 years and older. Vimkunya is a virus-like particle (VLP) vaccine. It utilizes virus-like particles (VLPs) that mimic the chikungunya virus to stimulate an immune response without exposing the recipient to the actual virus. Both received accelerated approval based on antibody studies. Neither vaccine has been demonstrated to reduce meaningful clinical outcomes in this disease.
Vimkunya Study 1 (n=3258) and Study 2 (n=413), solicited adverse reactions and collected them via electronic diary from the vaccination day through 7 days post-vaccination (an 8-day period). Unsolicited adverse events were monitored for 28 days post-vaccination. Serious adverse events were monitored through 6 months post-vaccination. New onset or worsening arthralgia that was medically attended was monitored through 6 months post-vaccination. Common side effects are similar to the illness including pain, muscle, and joint aches. No serious adverse events were reported.
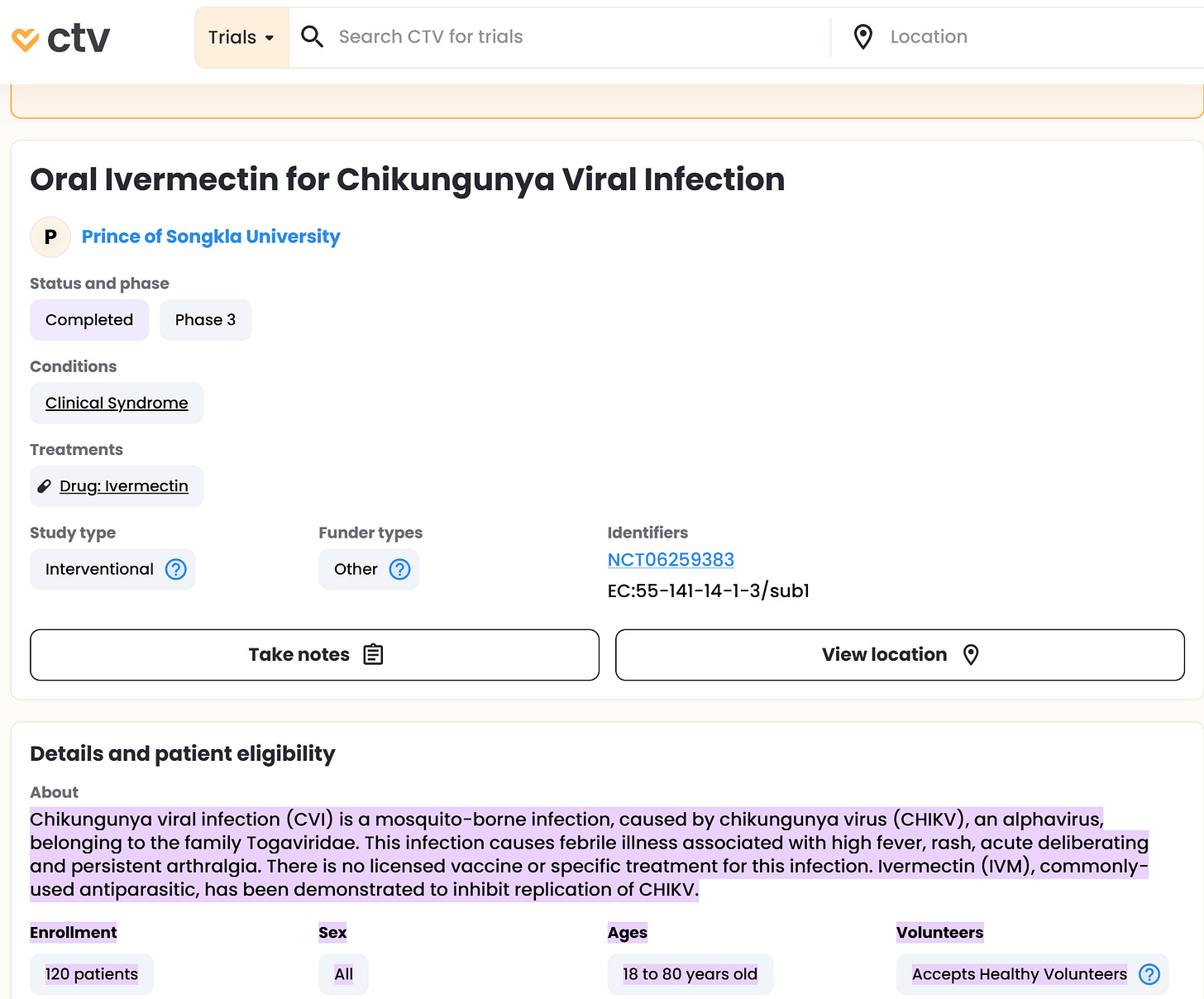
Ivermectin, a COVID-19 drug, has shown potential antiviral activity against the chikungunya virus in laboratory studies. Specifically, ivermectin has demonstrated the ability to inhibit viral replication in cell cultures, and studies suggest it may be most effective during the early stages of infection. Clinical trials are underway.
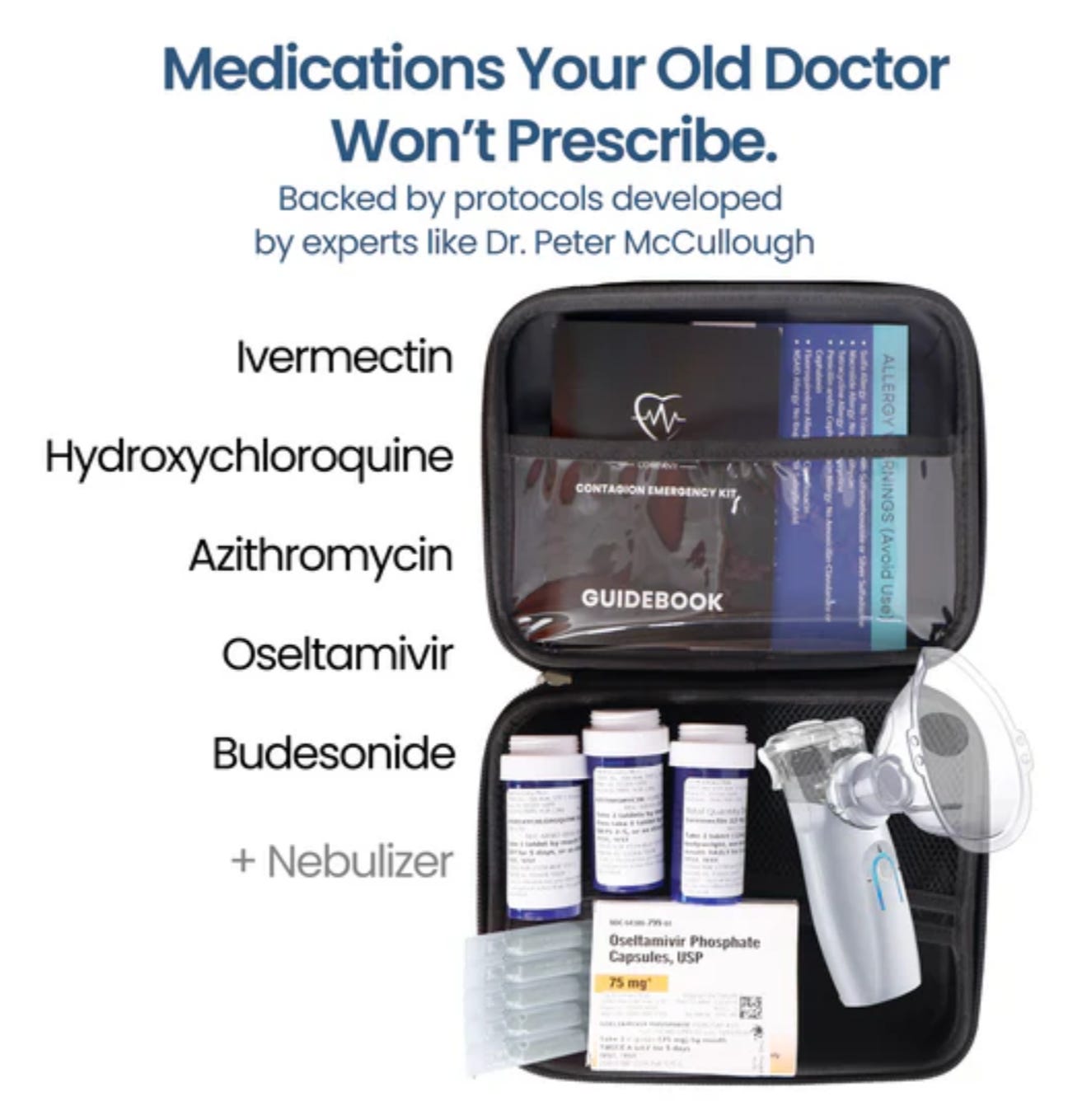
In the meantime it is reasonable in addition to good mosquito control to have a Contagion Kit from The Wellness Company on hand with ivermectin and secondary antibiotics if necessary.
Please subscribe to FOCAL POINTS as a paying ($5 monthly) or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
Chief Scientific Officer, The Wellness Company



The medical "scientists" who are creating these new for profit vaccines should test them for years on themselves, before marketing them.
One thing the SARS infection highlighted was the audit of your health it comprised. Poor health resulted in severe symptoms. Robust health resulted in a mild flu.
Is this not the case for all viral infections? Avian flu is severe when there is dioxin exposure. Is this true for humans and other viruses? It’s a good bet, especially given the global poisoning in play and a wide range of pollutants, in the air, water and food.
Control mosquitos … good idea. More bats and frogs needed: we’re hiring.
But measure and control pollution, at least as important.