Confounded Association Between Prenatal Tylenol and Childhood Neuropsychiatric Disorders
Large, Conclusive Swedish Study Finds Relationship, Demonstrates Lack of Independence
By Peter A. McCullough, MD, MPH
Before the recent HHS press briefing on autism, there was little or no discussion on mainstream, social, or Substack media on acetaminophen use during pregnancy. Many did not know Tylenol is one of many drugs implicated.
An AI search found at least thirty drugs used during pregnancy “linked” to neuropsychiatric problems later on in the child.
There are numerous prenatal drugs and substances that have been associated with increased risk of childhood neuropsychiatric or neurodevelopmental disorders (such as autism spectrum disorder, ADHD, intellectual disability, anxiety, depression, behavioral problems, or cognitive deficits) in the scientific literature. Based on a synthesis of peer-reviewed sources (including systematic reviews, cohort studies, and meta-analyses from PubMed, JAMA, BMJ, and other databases), at least 30 specific drugs have been associated with these risks to varying degrees. Citations are rendered inline where direct sources are available.
Anticonvulsants/Antiseizure Medications (5+ drugs) These are commonly linked to neurodevelopmental risks, especially autism and intellectual disability, due to interference with brain development.
Valproic acid/valproate: Strongly associated with ASD (up to 7-fold increased risk), lower IQ, and behavioral disorders.
jamanetwork.com +3
Carbamazepine: Neural tube defects and potential cognitive delays.
womensmentalhealth.org
Phenytoin: Linked to developmental delays and cognitive deficits (though evidence is weaker than for valproate).
pmc.ncbi.nlm.nih.gov
Topiramate: Increased risk of ASD and intellectual disability.
med.stanford.edu
Lamotrigine: Mixed evidence; some studies show weak links to oral clefts or learning difficulties, but often considered lower-risk.
aafp.org +1
Antidepressants (15+ drugs) Prenatal exposure, especially in the first trimester, has been linked to ASD, ADHD, altered brain development, and behavioral issues, though evidence is conflicting and often tied to underlying maternal depression.
SSRIs (selective serotonin reuptake inhibitors) as a class: Increased ASD risk (up to 2-fold) and altered pain response or stress axis function.
womensmentalhealth.org
Fluoxetine: Autism-like behaviors, lifelong behavioral abnormalities, altered serotonin function.
Paroxetine: Attention problems, aggression, hyperactivity.
Sertraline: Cognitive and behavioral changes.
Citalopram: Neonatal distress with potential long-term behavioral effects.
Escitalopram: Musculoskeletal defects and psychomotor delays.
TCAs (tricyclic antidepressants) as a class: Neonatal syndrome, long-term behavioral changes (e.g., altered social interaction, cognition).
Amitriptyline: Developmental delays, central nervous system effects.
Clomipramine: Autism-like responses, reduced anxiety in models.
Desipramine: Altered behavioral responsiveness.
Imipramine: Behavioral changes, altered brain histology.
Nortriptyline: Decreased body weight and potential developmental effects (animal models).
Trimipramine: Major abnormalities (animal models).
SNRIs (serotonin-norepinephrine reuptake inhibitors): Similar to SSRIs; disrupted behaviors.
Venlafaxine: Decreased exploratory/social behaviors.
Atypical antidepressants: Anxiety-like behaviors.
Bupropion: Increased anxiety, stress vulnerability, substance sensitivity.
Trazodone: Decreased exploratory/social behaviors.
MAOIs (monoamine oxidase inhibitors): Limited data, but linked to ASD.
Selegiline: Increased ASD risk.
Antipsychotics (7+ drugs) Associated with neurodevelopmental disorders and learning difficulties, though evidence is emerging and often for neonatal withdrawal rather than long-term effects.
Typical antipsychotics as a class: Potential congenital malformations.
Haloperidol: Teratogenic risks low, but neonatal effects.
Perphenazine: Malformations (low-potency agents).
Trifluoperazine: Similar to above.
Atypical antipsychotics as a class: Risk of specific neurodevelopmental disorders; neonatal extrapyramidal signs or withdrawal.
sciencedirect.com
Olanzapine: No major malformations, but neonatal complications.
Risperidone: Similar neonatal risks.
Quetiapine: Obstetrical/neonatal complications.
Clozapine: Limited data; potential malformations.
Aripiprazole: Limited data.
Opioids (4+ drugs)Linked to lower cognitive/motor skills, ADHD, and behavioral disorders, though not always substantial increases. bmj.com +4
Methadone: Lower mental development, neurodev impairment.
Morphine: Altered stress responses, anxiety-like behaviors.
Oxycodone: Similar to morphine; long-term morbidity.
Buprenorphine: Neonatal withdrawal, behavioral changes.
Other Medications (3+ drugs)
Acetaminophen: Increased risk of NDDs (e.g., autism, ADHD) and other neuropsychiatric disorders.
ehjournal.biomedcentral.com +1
Benzodiazepines (class): Possible increased risk of learning/neuropsychiatric disorders, cleft lip/palate (weak long-term data).
womensmentalhealth.org
Synthetic glucocorticoids (e.g., dexamethasone, betamethasone): Attention problems, executive dysfunction, cortical thinning.
pmc.ncbi.nlm.nih.gov
As an epidemiologist and a long-standing journal editor, I have become skilled at determining and examining the best and most conclusive sources of evidence among many publications on a topic. The reported link between prenatal acetaminophen use and the development of neuropsychiatric disorders several years later in the child is best evaluated by Ahlqvist et al, JAMA 2024.

Ahlqvist et al published an analysis from Sweden from a population-based sample of 2 480 797 children born in 1995 to 2019, with follow-up through December 31, 2021.
In total, 185 909 children (7.49%) were exposed to acetaminophen during pregnancy. Crude absolute risks at 10 years of age for those not exposed vs those exposed to acetaminophen were 1.33% vs 1.53% for autism, 2.46% vs 2.87% for ADHD, and 0.70% vs 0.82% for intellectual disability. In models without sibling control, ever-use vs no use of acetaminophen during pregnancy was associated with marginally increased risk of autism (hazard ratio [HR], 1.05 [95% CI, 1.02-1.08]; risk difference [RD] at 10 years of age, 0.09% [95% CI, -0.01% to 0.20%]), ADHD (HR, 1.07 [95% CI, 1.05-1.10]; RD, 0.21% [95% CI, 0.08%-0.34%]), and intellectual disability (HR, 1.05 [95% CI, 1.00-1.10]; RD, 0.04% [95% CI, -0.04% to 0.12%]). To address unobserved confounding, matched full sibling pairs were also analyzed. Sibling control analyses found no evidence that acetaminophen use during pregnancy was associated with autism (HR, 0.98 [95% CI, 0.93-1.04]; RD, 0.02% [95% CI, -0.14% to 0.18%]), ADHD (HR, 0.98 [95% CI, 0.94-1.02]; RD, -0.02% [95% CI, -0.21% to 0.15%]), or intellectual disability (HR, 1.01 [95% CI, 0.92-1.10]; RD, 0% [95% CI, -0.10% to 0.13%]). Similarly, there was no evidence of a dose-response pattern in sibling control analyses. For example, for autism, compared with no use of acetaminophen, persons with low (<25th percentile), medium (25th-75th percentile), and high (>75th percentile) mean daily acetaminophen use had HRs of 0.85, 0.96, and 0.88, respectively.
So it is true in the unadjusted analysis that women who took acetaminophen did have higher rates of affected children, but when known confounders were included in the multivariate analysis, which is the main tool used when randomization was not possible, there was no association.
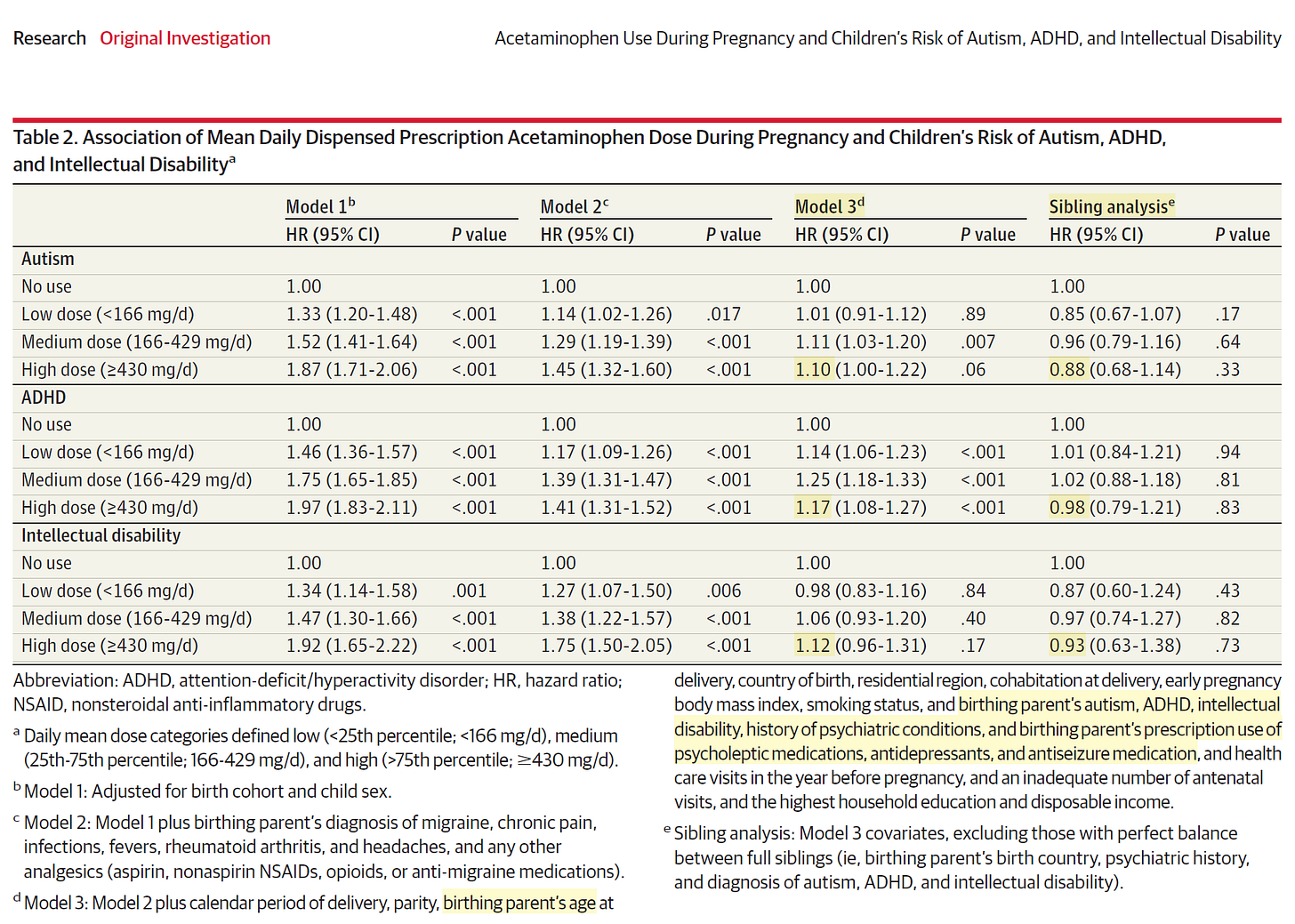
The key to understanding Ahlqvist as a valid source of evidence is Table 2 depicting the multivariate analysis controlling for other possible determinants of neuropsychiatric disorders: age of parents, early delivery, birthing parent’s autism, parental ADHD, intellectual disability, history of psychiatric conditions, and parent’s use of psycholeptic medications, antidepressants, and anti-epileptic medication. Note that acetaminophen at medium and higher doses (means it was taken on most days during nine months) as determined by structured nurse-midwife assessment had modest <20% excess risk that remained statistically significant for autism, ADHD, and intellectual disability. None of this was confirmed by examining siblings born to the same parents where one was exposed to acetaminophen and the other was not. A common scenario listed in the supplemental materials where a woman took acetaminophen during one pregnancy and not in another was migraine headaches.
At the McCullough Foundation we have developed a track record of being a true north on contemporary issues and we are not afraid to agree with organizations for which on other issues we may have disagreed.
We agree with least 7 medical societies have issued statements affirming that acetaminophen is safe for use during pregnancy (when taken as directed or judiciously) and is not a cause of autism in children.
American College of Obstetricians and Gynecologists (ACOG): Affirms that “acetaminophen remains the safest first-line analgesic and antipyretic in pregnancy” and “the current weight of evidence does not support a causal link between prenatal acetaminophen use and neurodevelopmental disorders.”
acog.org +1
Society for Maternal-Fetal Medicine (SMFM): States that “acetaminophen use during pregnancy has not been shown to cause or increase the risk of autism” and recommends it as an “appropriate medication” for pain and fever, noting untreated conditions carry significant risks.
smfm.org +2
American Academy of Pediatrics (AAP): Supports that “the occasional use of acetaminophen as directed for fever and pain relief during pregnancy is safe” and “studies do not point to a causal link between the use of acetaminophen and autism in children or in pregnancy.”
aap.org +1
National Medical Association (NMA): Describes claims of a link as “misleading” and affirms that “acetaminophen has long been used safely in pregnancy, including for the treatment of high fevers.”
nmanet.org
International and Non-US Societies
International Federation of Gynecology and Obstetrics (FIGO): Recognizes paracetamol (acetaminophen) as “safe during pregnancy when clinically indicated” and states that evidence “does not support a causal association” with autism.
figo.org
Royal College of Obstetricians and Gynaecologists (RCOG, UK): Recommends paracetamol as the “first-line analgesic during pregnancy,” aligning with the consensus that it is safe and not causally linked to neurodevelopmental disorders.
figo.org
Society of Obstetricians and Gynaecologists of Canada (SOGC): Recommends acetaminophen as a “first-line” option and states it “remains a safe and appropriate option for managing fever and pain during pregnancy when used at the lowest effective dose.”
sogc.org +1
Inclusion, there is a bundle of prenatal exposures in which acetaminophen is included that do elevate the risk for a child developing a neuropsychiatric disorder. Based on this comprehensive analysis a reasonable conclusion is that acetaminophen alone taken during pregnancy is not a cause of autism that develops years later in an afflicted child.
Please subscribe to FOCAL POINTS as a paying ($5 monthly) or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
FOCAL POINTS has partnered with Patriot Mobile to defend your medical freedom. Join Patriot Mobile today!




I read the Swedish study (Ahlqvist et al.) carefully - the full article, not just the abstract, particularly looking at the "methods" - and it looked to me like they did not truly know how much Tylenol was even taken by the mothers. When Tylenol was prescribed by a physician, it was documented how many tablets the woman received from the pharmacy, but there was no determination of how much she truly took. For myself, I have numerous bottles of prescribed meds at home that I have never taken - for example, Tylenol with Codeine prescribed for dental work but I never took any of the medication; or Tylenol with codeine after a surgical procedure, when I only needed one tablet but received a dozen. A muscle relaxer that I never took after I read the side effects, though it was filled twice because my husband picked it up on my behalf, not realizing I didn't want it. So in this study - just looking at how many tablets were filled is not, in my opinion, a reasonable way to know how much Tylenol was taken, if any was even taken. They also considered that women may purchase nonprescription Tylenol that was not filled at the pharmacy, but from what they wrote it sounded like they really had not done a good job accounting for these either. If they don't know how much Tylenol was taken, or even if Tylenol was taken at all, the study is meaningless, even though it looks great from the abstract. But I think there methods of accounting for actual Tylenol use were very poor. I can't quote the article directly right now because I don't have access at the moment. My email account at the college where I teach got hacked last week and my password was frozen by IT who have not restored my account yet. But I read this carefully several days ago.
Even if you assume that everyone who filled a prescription truly did take some, it matters how much the mother took, because part of the criteria for determining causality (the Bradford Hill criteria) says that you would expect, if a med caused some problem, your chance of having the problem is greater if you took more of the meds, compared to someone else. So how much the woman took and of course knowing for sure if she took it at all, is important information that I don't think is accurately known from the methods used.
On the other hand, a different study, published August 14, 2025, analyzed 46 different studies on the association between Tylenol used by the mothers and autism and ADHD in the children. This is the study: Prada, D., Ritz, B., Bauer, A.Z. & Andrea Baccarelli. Evaluation of the evidence on acetaminophen use and neurodevelopmental disorders using the Navigation Guide methodology. Environ Health 24, 56 (2025). https://doi.org/10.1186/s12940-025-01208-0. And This study by Prada et al., , and found the majority of these studies showed a strong effect, plus also a dose effect, in that mothers who took Tylenol for more than four weeks, had the highest risk of having a child with autism or ADHD, a greater risk compared to mothers who took it for a shorter period of time.
According to the Bradford Hill criteria for causality, when you see an association & hope to figure out, is the one thing causing the other, you look at consistency - is this happening over and over in different studies? In this case, out of 46 studies, the majority showed this association, not just one or two. It also should be strong association, not a tiny, iffy one - and in this case, that was true. Then, the dose-response as I mentioned, which was present - the children of the women who took Tylenol for 4 weeks or longer had greater risk of having ADHD or autism. And then, is it plausible, is there any realistic explanation that is logical that shows how this could happen. And in fact, in our bodies, we all make glutathione which helps our bodies to inactivate harmful chemicals that we may be exposed to. But Tylenol depletes glutathione & leaves people more vulnerable to toxins. Tylenol itself is toxic, and is known to cause many cases of liver damage and even liver failure every year. It can damage cells & not only liver cells.
Because babies in the womb during weeks 4 through 8 have all their organs and organ systems rapidly developing during that time, that is a time of special vulnerability when exposure to any chemical might cause damage to the developing organ even something that might not bother at all later on. So, partly it may make a difference at what point in the pregnancy the mom took a medication. As pointed out in Dr. McCullough's article, many meds are associated with various problematic outcomes. It's good to minimize any meds that you don't have to take during pregnancy. But in the US, it is apparently recommended that women take 4 vaccines during pregnancy, one of them being the flu shot. The flu shot, or at least some flu vaccines, have aluminum in them, which is a neurotoxin. Aluminum can cross the placenta, and it can get into the babies brain. But if the mother takes Tylenol due to a vaccine reaction, maybe a fever or discomfort after the vaccine, she is depleting her glutathione which makes her then less able to inactivate various toxins in her body - so this has been proposed as a plausible mechanism by which Tylenol could be causing harm.
In the end, I think that probably the in utero exposures are not really the most important concern, but with all the aluminum that has been in the vaccines until about now, and kids getting repeated doses of aluminum from infancy through adulthood, and again, aluminum being a neurotoxin, the bigger concern might be all the aluminum that has been injected into children and then the infants or children being given Tylenol after the shots. Hopefully we'll get greater clarity on that soon with some of the new research that is anticipated to be available soon.
First of all, I don’t trust all the alphabet soup agencies you agree with. They have proven time and again to be untrustworthy. No one cares what they say anymore.
Secondly, the manufacturer itself was concerned about a link with autism, according to leaked emails, and did what Pharma always does and sweep it under the rug. That is concerning.
However, I still do not believe the link is strong. Why? Because nearly every pregnant woman probably takes Tylenol. They are told by their “trusted” doctors that it is the ONLY safe pain reliever/fever reducer to take during pregnancy. This has been true for decades. Thankfully, I did not take it during my two pregnancies over 2 decades ago, because I would not take any medications.
What is being missed in focusing on taking Tylenol during pregnancy, is that it is much more harmful to give to an infant. I remember 30 years ago, those in the holistic community warning about giving Tylenol after vaccines. Which again, is exactly what doctors tell parents to do when they spike a fever after vaccination. If I remember correctly, they believed it caused a Cytokine Storm which caused damage to the brain.
Are there any studies on giving Tylenol to infants after vaccination?
There are already thousands of parents who believe their child regressed into autism after routine vaccinations. It would be interesting to know how many of those children took Tylenol as well.