FDA Recommends More mRNA Shots Despite New Study Showing Booster Failure—and Major Public Outcry
FDA VRBPAC ignores new real-world study of 1.9 million showing mRNA booster campaign failure—and over 90,000 public comments voicing serious concerns.
On May 22, 2025, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) convened to evaluate and recommend the formulation of COVID-19 vaccines for the 2025–2026 season, set to begin in the fall. In anticipation of this meeting, the public submitted over 90,000 comments detailing their serious concerns over continued mRNA injection campaigns.
Despite major public concern, the committee unanimously endorsed a monovalent JN.1-lineage vaccine (LP.8.1 strain)—signaling a continued push for mRNA injection campaigns.
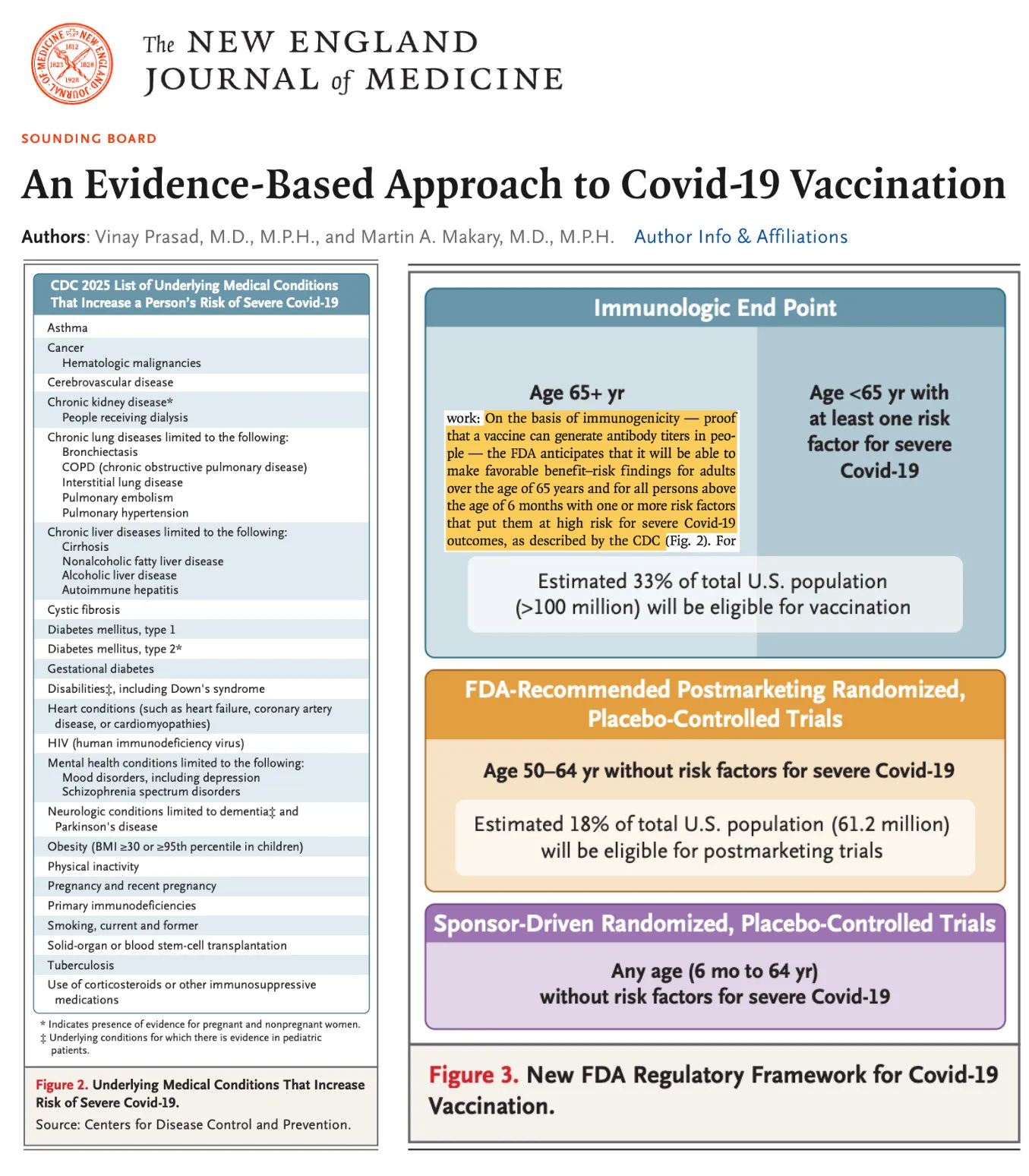
This outcome was anticipated given the recent FDA policy statement in The New England Journal of Medicine, which reveals that millions of so-called “high-risk” (pregnant women, 6-month-old infants with diabetes, etc.) Americans will remain subject to mRNA booster recommendations based solely on immunogenicity data:
The fact that the FDA’s VRBPAC disregarded the voices of nearly 100,000 concerned Americans comes as no surprise—especially given that their December 11, 2020 decision to grant emergency use authorization for Pfizer’s mRNA injection was later deemed invalid by the DailyClout investigative team. Their investigation uncovered multiple deliberate mechanisms of trial deception, including underreporting deaths, concealing autopsy findings, and manipulating efficacy timelines to mislead the public:
A few days before the May 22, 2025 VRBPAC meeting, a new study was published by Schaffer et al in the journal Vaccine—and it directly undermines the rationale for continued mRNA booster campaigns.
The researchers evaluated the effectiveness of the 2022 autumn COVID-19 booster rollout in England among over 1.9 million adults aged 45–54. Their primary outcome: whether the campaign reduced COVID-19 emergency visits, hospitalizations, or deaths.
The verdict was clear: the booster campaign failed.
The estimated reduction in severe COVID-19 outcomes among 50-year-olds was a negligible −0.4 events per 100,000, with a wide confidence interval (−7.8 to 7.1) that renders the result statistically insignificant and clinically meaningless. Even among actual recipients (“compliers”), the study reported a positive risk difference of 2.1 per 100,000—indicating more severe outcomes in the boosted group, though this too was not statistically significant (95% CI: −11.3 to 15.4).
In conclusion, the FDA’s decision to press forward with yet another mRNA booster campaign—despite overwhelming public opposition, clear evidence of failure, and a growing mountain of data linking these shots to catastrophic harm and death—reveals a deeply troubling pattern of policy separated from reality. It is long past time for regulators to abandon failed strategies and prioritize honest, evidence-based public health.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.








Where is RFK?????? He knows this! He needs to speak up!!
They don’t care and no one can stop them! Unbelievable!