Dr. Jeyanthi Kunadhasan is an Australian anesthesiologist and perioperative physician who was terminated from her hospital position after questioning the risk-benefit profile of COVID-19 vaccines in healthy individuals. Following her dismissal, she joined a group of international medical volunteers tasked with reviewing the 500,000+ pages of internal Pfizer documents released as part of a court-ordered FOIA request. This effort was coordinated by DailyClout and Dr. Naomi Wolf. As a result of that work, Dr. Kunadhasan has become one of the foremost experts on Pfizer’s pivotal mRNA vaccine trial, focusing specifically on discrepancies in reported deaths and adverse events.
In this episode of Focal Points, Dr. Peter McCullough and epidemiologist Nicolas Hulscher are joined by Dr. Jeyanthi Kunadhasan, who presents her independent forensic analysis of Pfizer’s COVID-19 vaccine trial data. Her findings reveal that multiple vaccine-related deaths were concealed from regulators, autopsy results were either buried or never conducted, and Pfizer appeared to deliberately delay its efficacy announcement until after the 2020 U.S. presidential election. The December 10, 2020 FDA VRBPAC Meeting that emergency use authorized (EUA) the Pfizer-BioNTech COVID-19 Vaccine (also known as BNT162b2) are shown in this image. Most have shunned the public spotlight after this fateful meeting that reviewed interim (not final) clinical trial data.
What Dr. Kunadhasan uncovers raises grave questions about data integrity, regulatory failure, fraud, and the validity of the FDA’s Emergency Use Authorization for the Pfizer-BioNTech COVID-19 vaccine. Everyone at the meeting either knew or should have known to ask about deaths not included in the core slides and briefing booklet.
A Falsified Safety Narrative
What the Pfizer reported in the FDA VRBPAC core slides and briefing booklet from interim study data through of Nov 14, 2020:
6 total deaths:
2 in the vaccine group
4 in the placebo group
What actually occurred:
11 total deaths:
6 in the vaccine group
5 in the placebo group
Pfizer failed to disclose four vaccine deaths in the core slides and briefing booklet. Two of these deaths had already been reported by family members to clinical trial sites before the cutoff date—meaning Pfizer was obligated by regulatory standards to report them to the FDA. They did not. From November 14, 2021 to the FDA VRBPAC meeting on Dec 10th, there were an additional 6 deaths. (2 additional vaccine deaths, 4 placebo). This means if anyone had asked Pfizer to update the panel on deaths not listed in the slides or briefing booklet, Pfizer should have disclosed 8 vaccine deaths and 9 placebo deaths. But no one asked.
Buried Autopsies, Sudden Deaths, and Suppressed Evidence
Two vaccine deaths in particular stand out:
Subject 11141050 (Kansas):
63-year-old woman
Autopsy-confirmed sudden cardiac death
Family notified the site on October 19, 2020
Pfizer failed to enter the death until after the November 14 data cutoff
Autopsy result was altered in the case report form to “unknown”
Subject 11201050 (Georgia):
58-year-old woman
Died in her sleep on November 7, 2020
Clinical site notified the same day
No autopsy performed
Dr. Kunadhasan’s analysis revealed:
10 sudden adult deaths in the vaccine arm
Only 2 autopsies performed
Of those, one was concealed and one remains with no results available
356 Trial Participants Lost to Follow-Up
From the paper by Michels et al, a total of 203 subjects were lost to followup prior to Nov 14th 2020 (99 vaccine, 104 placebo). As reported by Thomas et al, NEJM September 15, 2021, Pfizer lost contact with 356 participants, compromising the trial’s statistical integrity. Additionally, another 447 withdrew from the study without mention of follow-up data. Thus, 803/44,165 (1.8%) had incomplete information on safety and efficacy. With endpoints of SARS-CoV-2 infection occurring in only 927/42,094 (2.2%) of evaluable subjects, the trial results were not robust to missing data. Dr. McCullough emphasized this would be disqualifying in any cardiovascular trial.
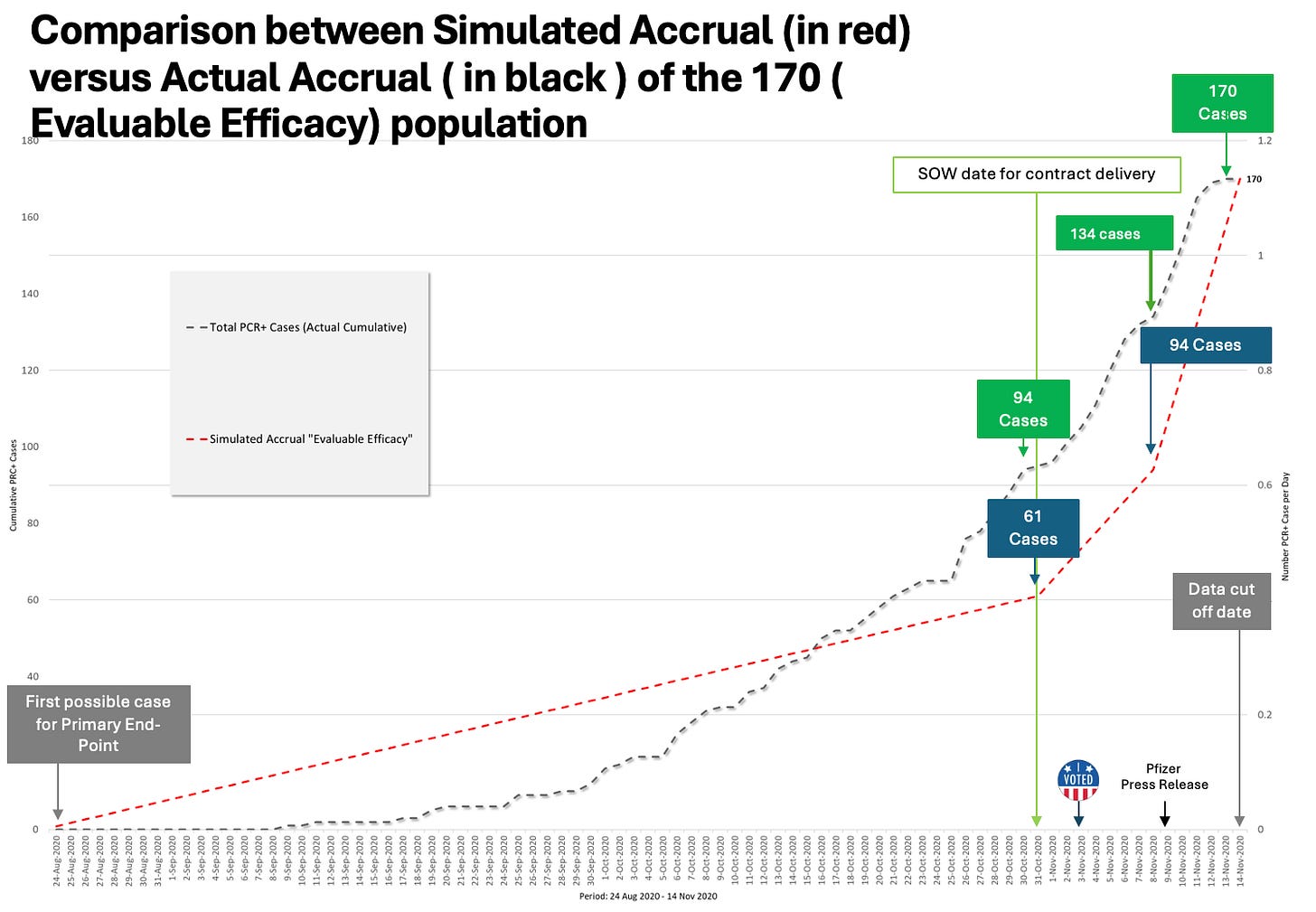
Delayed Efficacy Analysis to Avoid Pre-Election Announcement
According to internal documentation and public records:
Pfizer had agreed via contract with the Trump administration to deliver an “efficacious” vaccine by October 31, 2020
The efficacy threshold could have been reached as early as October 9, when 100% of COVID cases were in the placebo group
Instead of analyzing efficacy at the agreed 32-case or 62-case threshold, Pfizer delayed its analysis until November 8—after the 2020 election
At that point, Pfizer announced 94 cases and declared 95% efficacy. But Dr. Kunadhasan’s team found 134 eligible cases had actually accumulated—40 more than reported.
Legal Action
Dr. Kunadhasan has submitted formal letters and evidence packages to:
A few days ago, Rep. Jim Jordan launched a major probe into the timing of Pfizer's vaccine announcement. Also, Kansas Attorney General Kris Kobach’s case filed against Pfizer for false COVID vaccine marketing is to be heard at state level.
Conclusion
This investigation exposes five distinct mechanisms of trial deception:
Underreporting vaccine deaths at the EUA cutoff
Burying autopsy-confirmed fatalities
Delaying efficacy data beyond the 2020 election
Losing hundreds of patients to follow-up
Misclassifying causes of death to obscure safety signals
These findings—based on public documents and direct correspondence—indicate that the FDA’s authorization of Pfizer’s mRNA vaccine was based on incomplete, manipulated, and selectively reported data, and is therefore invalid.
This fraudulent authorization likely resulted in at least 470,000 American deaths:
Accountability and justice for this historic travesty are urgently warranted.
For a comprehensive analysis of Pfizer’s internal documents and clinical trial data, see The Pfizer Papers: Pfizer's Crimes Against Humanity.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.