NEW STUDY: Fenbendazole Linked to Remission or Near-Remission in Three Stage IV Cancer Patients
Breast, prostate, and melanoma patients experienced dramatic tumor regression and long-lasting remission — without chemotherapy.
Fenbendazole (FBZ) is a low-cost veterinary antiparasitic drug that has gained global attention as a potential anticancer therapy. Like ivermectin—another antiparasitic widely repurposed in research and clinical practice for its potent anti-tumor effects—fenbendazole appears to work far beyond its original veterinary use.
Preclinical studies show fenbendazole disrupts cancer cell survival through multiple pathways, but until now, human evidence has been scarce.
A newly published case series by Dr. William Makis et al in Case Reports in Oncology presents three remarkable patients with advanced, stage IV cancers (breast, prostate, and melanoma) who self-administered Fenbendazole outside conventional oncology protocols. All three achieved either complete or near-complete remission — sustained for up to three years — without chemotherapy:
Case 1 – Stage IV Breast Cancer
Patient: 83-year-old woman with widely metastatic ER/PR-positive, HER2-negative breast cancer involving the liver, lungs, and spine.
Treatment: Daily FBZ (222 mg), fulvestrant (estrogen blocker), brief targeted radiation for spinal lesions, vitamin D and multivitamins.
Outcome: Within 8 months, tumor markers normalized and PET scans confirmed no evidence of disease. She has remained recurrence-free for nearly 3 years on continued FBZ with no adverse effects.
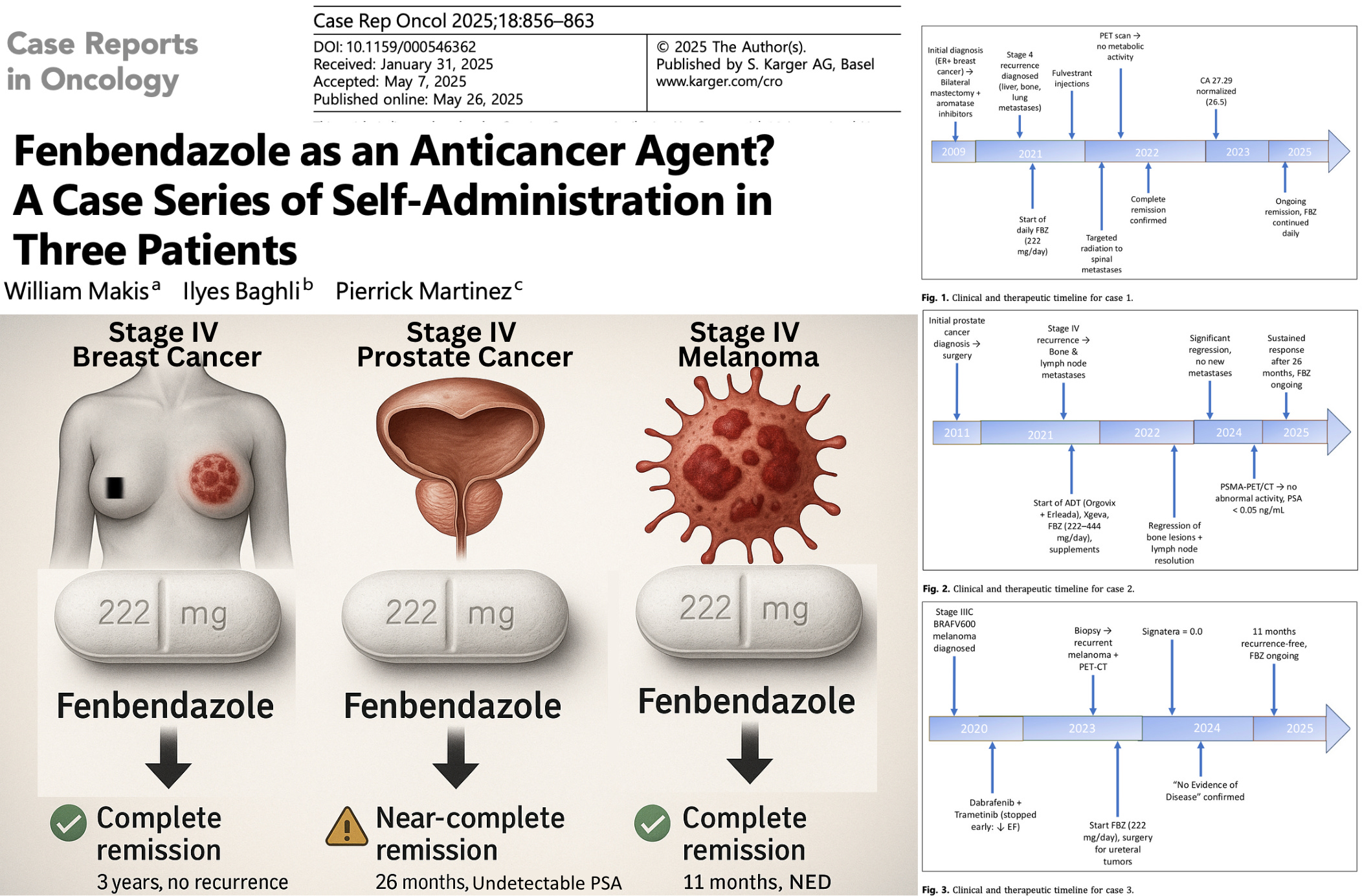
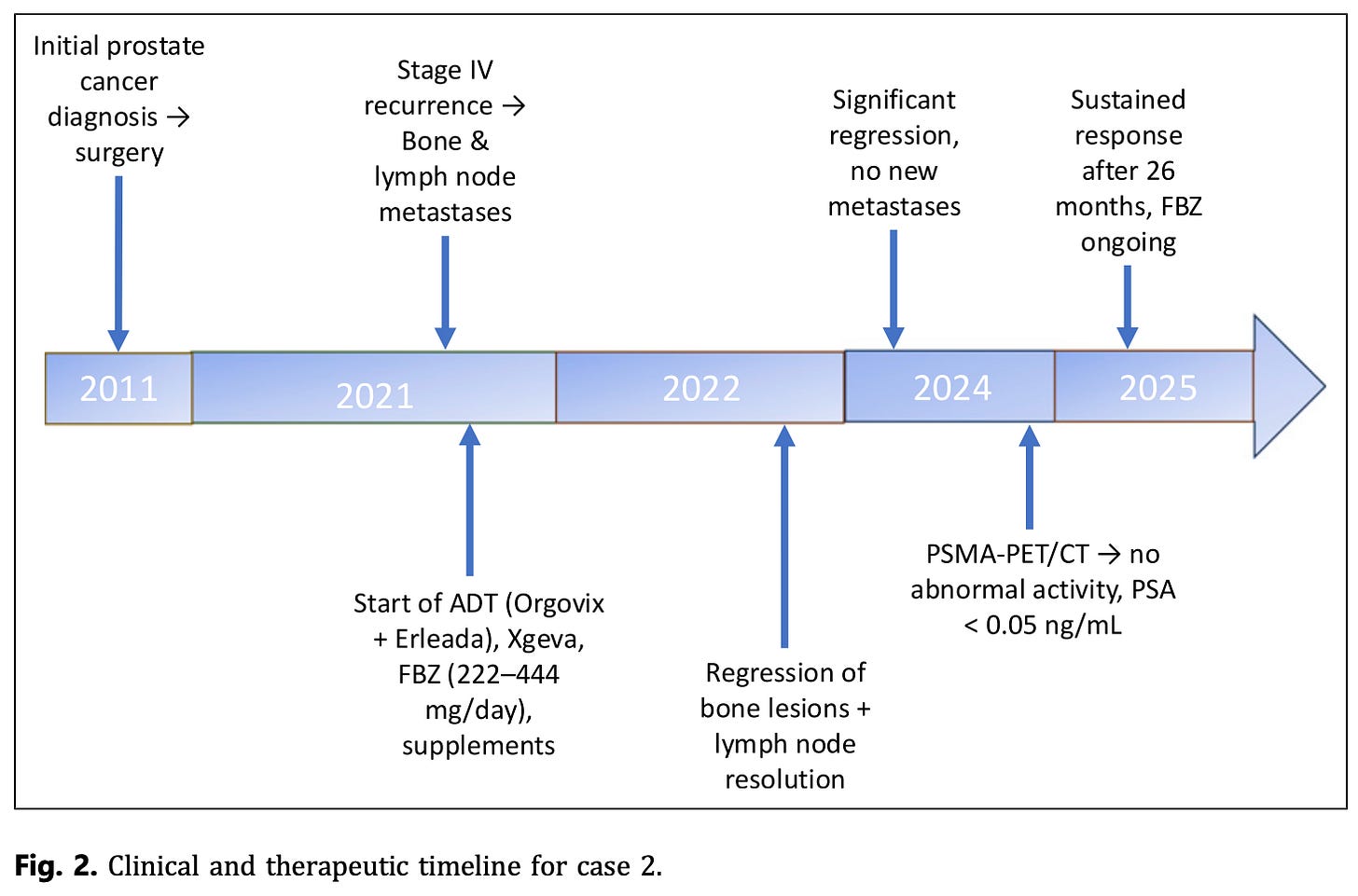
Case 2 – Stage IV Prostate Cancer
Patient: 75-year-old man with recurrent metastatic prostate cancer (spine, pelvis, humerus, and lymph nodes).
Treatment: Androgen deprivation therapy (Orgovix, Erleada, Xgeva) plus supplements (vitamin D/K2, melatonin, berberine, curcumin, artemisinin). Added FBZ 222–444 mg/day.
Outcome: Regression of bone and lymph node metastases. PSA remained undetectable for over 2 years. He is in a near-complete remission at 26 months follow-up, continuing FBZ with ADT.
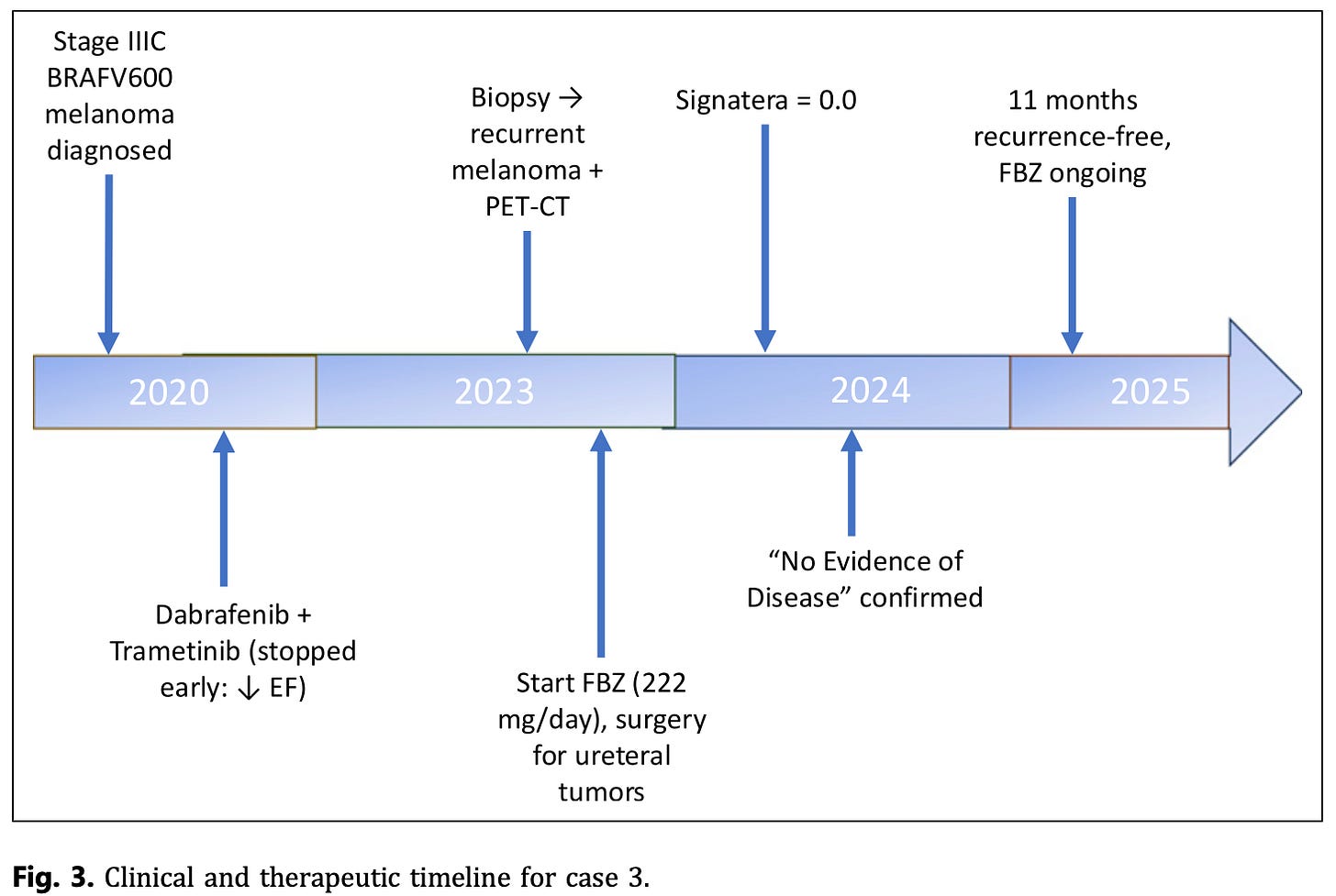
Case 3 – Stage IV Melanoma (BRAFV600+)
Patient: 63-year-old man with recurrent metastatic melanoma, also diagnosed with a ureteral carcinoma.
Treatment: Began FBZ (222–444 mg/day) during a treatment gap, later received two doses of nivolumab. Also took routine supplements (vitamin C, D, CoQ10, B12, glutathione).
Outcome: Circulating tumor DNA dropped from 123 → 0 in under 2 months. Imaging confirmed complete remission with no evidence of disease. Patient remains recurrence-free 11 months later.
MECHANISMS OF ACTION
As outlined in this case series, preclinical studies on fenbendazole reveal multiple anticancer mechanisms that may explain the dramatic outcomes seen in these cases:
Microtubule destabilization → FBZ binds tubulin, blocking polymerization, leading to mitotic arrest and apoptosis (similar to chemotherapy agents like vinca alkaloids).
p53 activation → triggers mitochondrial apoptosis and DNA damage response, selectively killing cancer cells.
Proteasome inhibition → interferes with protein degradation, suppressing tumor cell proliferation.
Metabolic disruption → inhibits glucose uptake and likely glutamine utilization, starving cancer cells of essential fuel.
Anti-angiogenesis → reduces tumor blood vessel formation, limiting nutrient supply.
Cancer stem cell effects → FBZ and other benzimidazoles appear capable of suppressing resistant tumor-initiating cells.
This is only the second published case series documenting human cancer remissions linked to fenbendazole use. Strikingly, two of the three patients achieved “no evidence of disease” — a rare outcome in advanced stage IV cancers.
However:
Patients self-medicated without controlled oversight.
All used FBZ alongside other treatments, making causality unclear.
Spontaneous remission, though rare, cannot be excluded.
Still, the consistency across cases — different cancers, different regimens, same outcome: regression or remission — signals a phenomenon too important to dismiss.
FBZ is cheap, accessible, and already proven safe in veterinary medicine. What’s lacking is rigorous human clinical research. Clinical trials should be immediately inititated to assess fenbendazole’s true potential.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.




Unfortunately we know what happens when you do the research and prove Fenbendazole's effectiveness. The FDA comes in and bands it from the marketplace. Healthy people aren't profitable.
What an incredible service you provide with your posts. I really appreciate your participation in, and highlighting, of clinical research. It's so important. I've added this and the research you reference to the curation of published research on cancer reversal and recovery.
We're now up to 42 references on anti-parasitics + 147 references on other successful treatments:
https://birdseyeviewperspective.substack.com/p/cancer-reversal-and-recovery-successes