I sat down with Dr. Carol Taccetta—a physician with decades of experience in drug safety who contributed to the Surgeon General’s landmark report on smoking—to unpack why, despite overwhelming evidence of fatal myocarditis linked to the COVID-19 mRNA shots, the FDA continues to deny not only a market recall, but even the bare minimum: a black box warning.
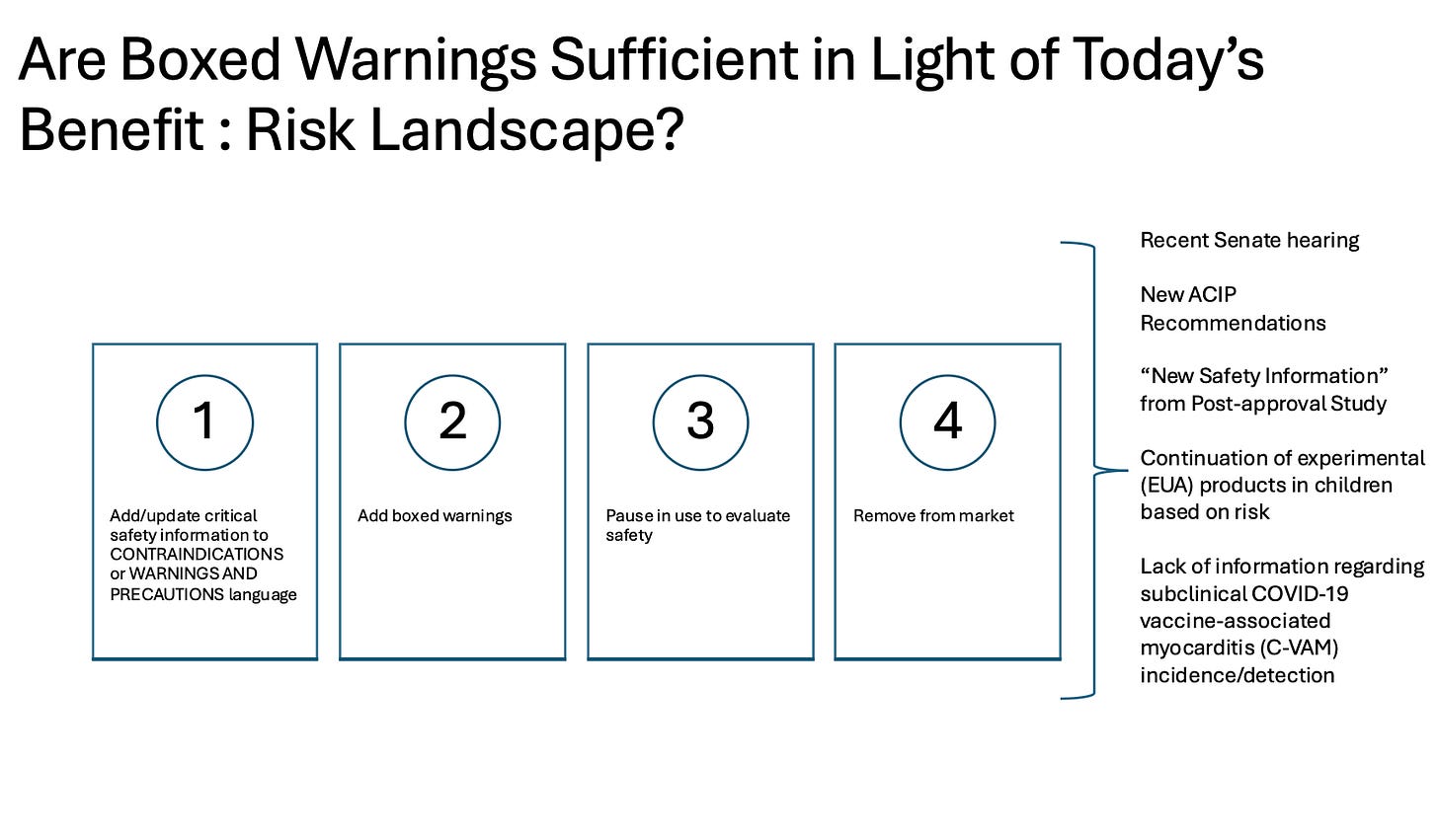
What Is a Black Box Warning—And Why Does It Matter?
Dr. Taccetta explains that a black box warning is the FDA’s most serious labeling requirement. It's reserved for risks that are fatal, life-threatening, or permanently disabling—and when proper warnings can reduce harm. The warning must be placed front and center on prescribing information, especially when the risk can be mitigated by monitoring, patient selection, or dose adjustment.
The question isn’t whether myocarditis qualifies—it clearly does—but why the FDA is refusing to act.
The Evidence Is Overwhelming
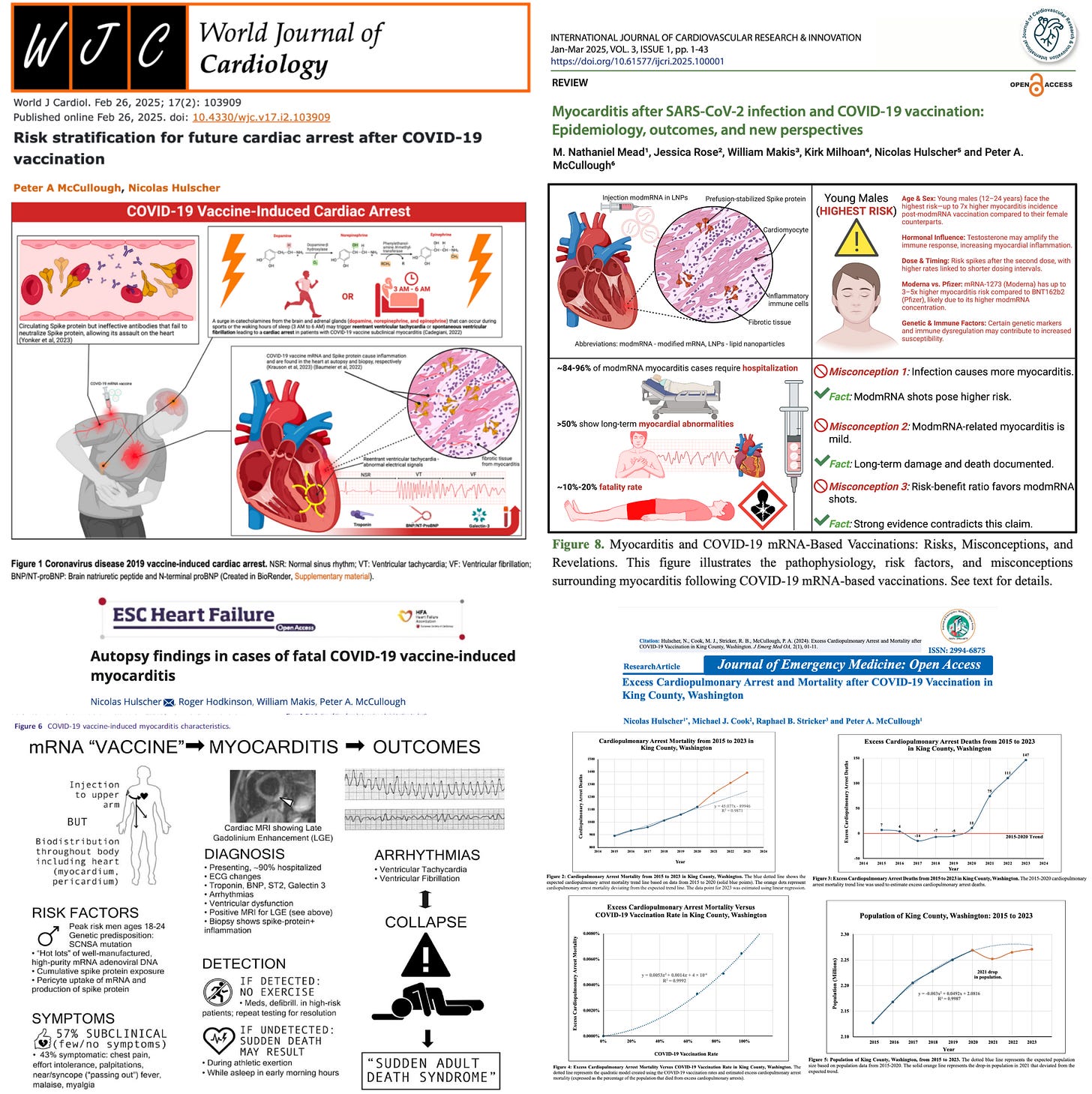
From autopsy findings to population-based studies, the signal couldn’t be clearer: vaccine-induced myocarditis can be fatal:
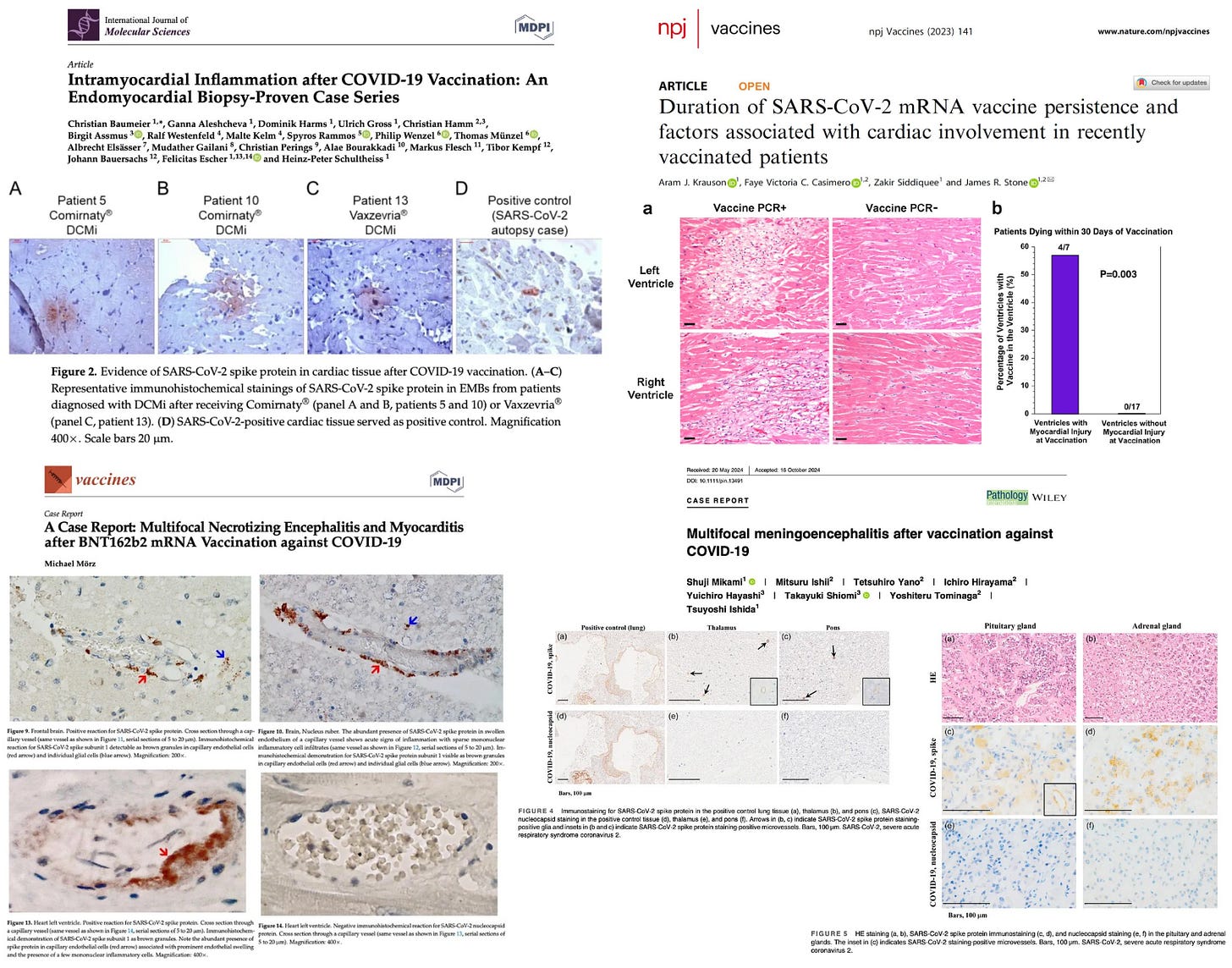
Both mRNA and spike protein have been detected in cardiac tissue months after injection in the injured and deceased:
Dr. Taccetta further pointed to CDC admission of a “likely association” and VAERS data showing over 500 myocarditis-linked deaths. That alone should have triggered the highest safety alert years ago.
The FDA's Excuses Don't Hold Up
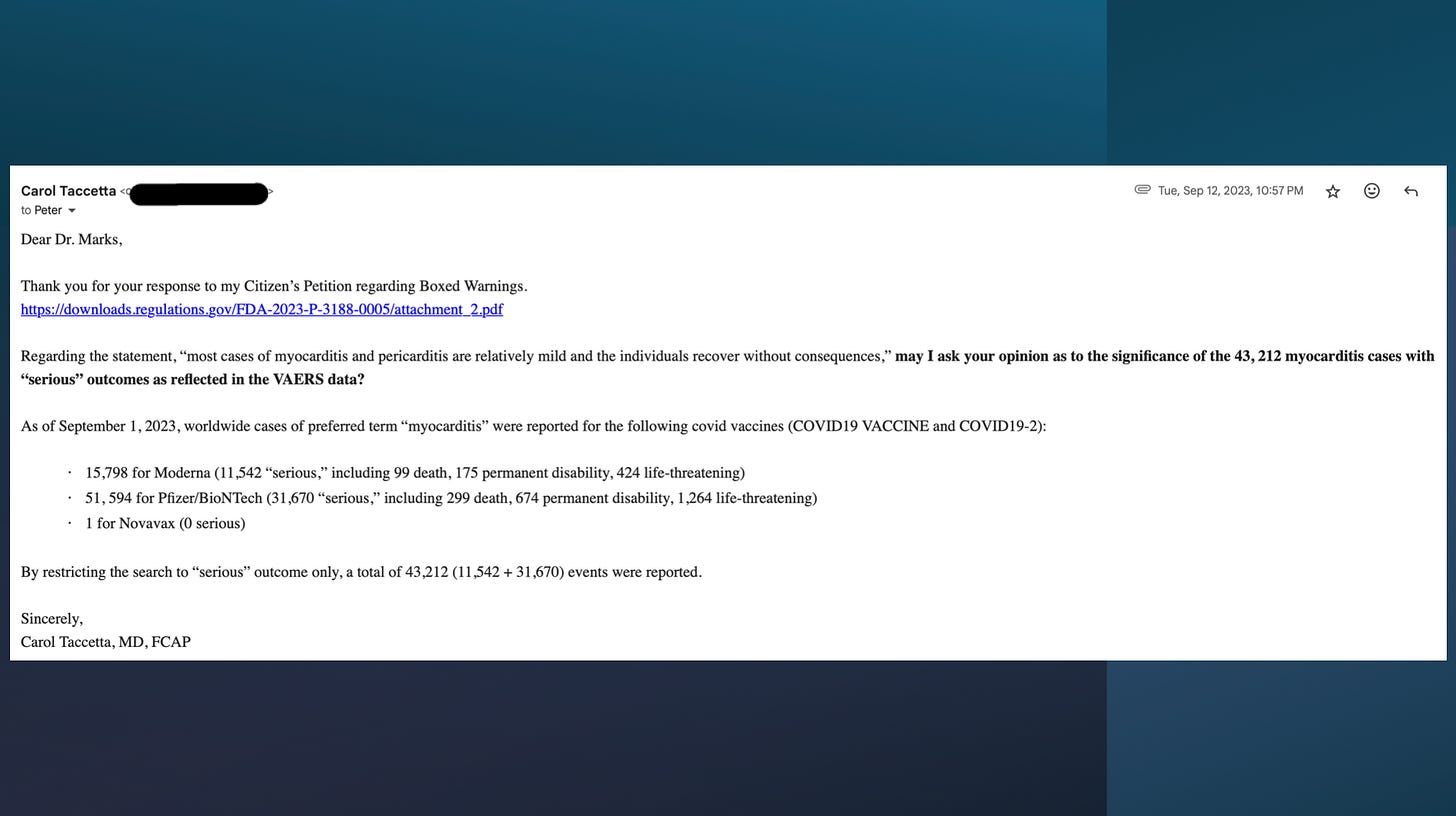
When Dr. Taccetta filed her citizen petition in 2023—backed by DailyClout and informed by their internal research team—she formally requested black box warnings for myocarditis and anaphylaxis on the COVID-19 mRNA injections.
The FDA denied it. Their excuse was that “Most cases are mild.”
They ignored the 43,000+ serious adverse events she documented. They dismissed autopsy findings, CMR imaging studies showing persistent cardiac scarring, and even their own insurance claims data. The agency simply refused to act on the overwhelming body of evidence presented.
This is the same FDA that recommended a pause to the Janssen vaccine for just 6 cases of thrombosis with thrombocytopenia syndrome (TTS). Yet now they claim no action is needed despite at least tens of thousands of serious heart injuries and deaths.
New FDA Labeling Changes: Too Little, Too Late
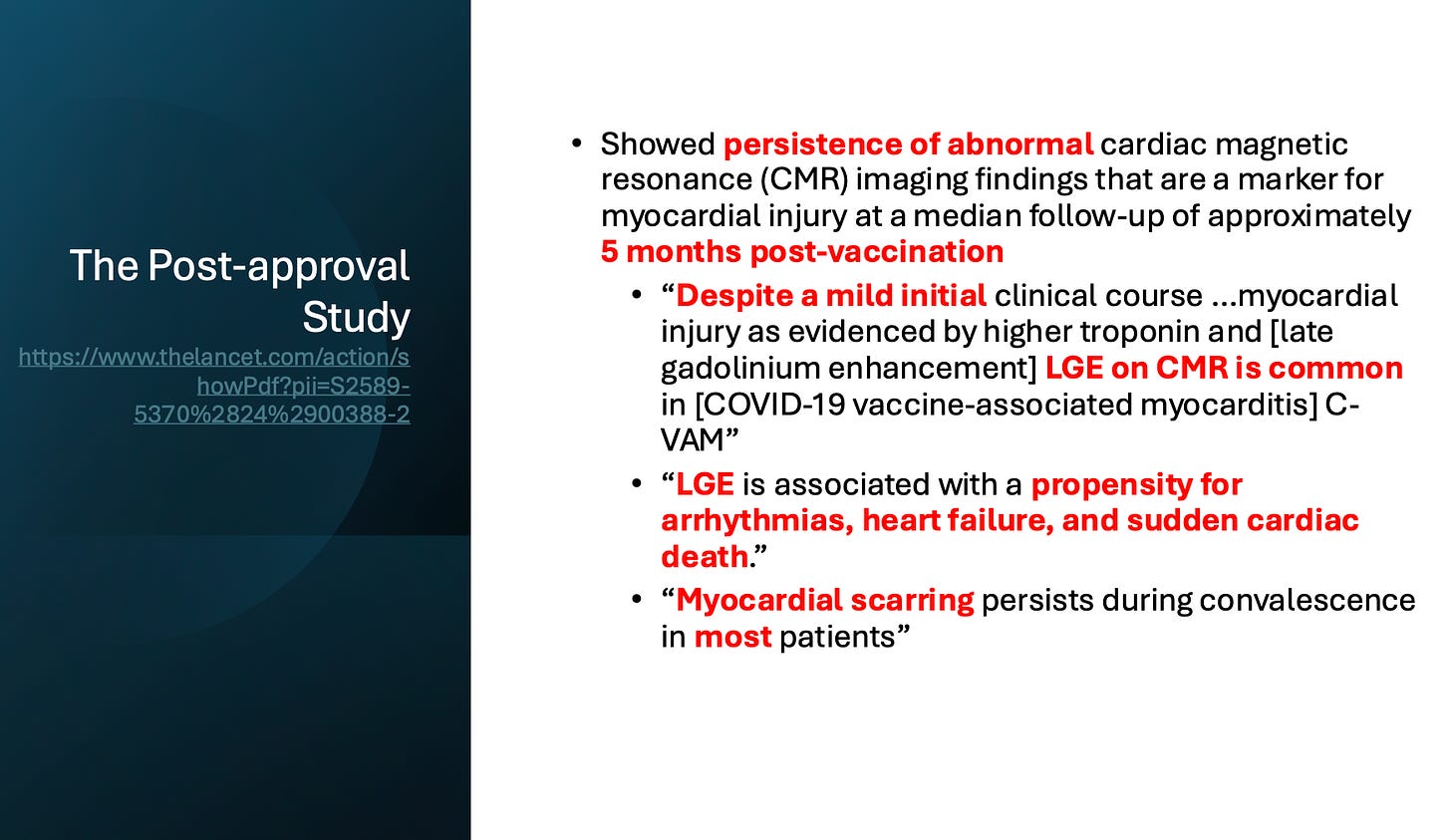
In May 2025, the FDA acknowledged more risks—but only through vague label tweaks.
They admitted persistent cardiac injury in post-vaccine myocarditis cases. But they refused to include the terms death, heart failure, or arrhythmia risk—even though the study they cited links vaccine heart injury to sudden cardiac death:
Dr. Taccetta has spent countless hours trying to make a difference—submitting petitions, engaging the process, and writing directly to Dr. Peter Marks—all in an effort to fully inform the public about the risk of fatal heart damage.
But the FDA’s continued denial of black box warnings, even after confirming long-term cardiac injury, makes one thing clear: this agency has abandoned its duty to protect the public.
We don’t just need better labels—we need these shots off the market. Until then, we will keep sounding the alarm.
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.