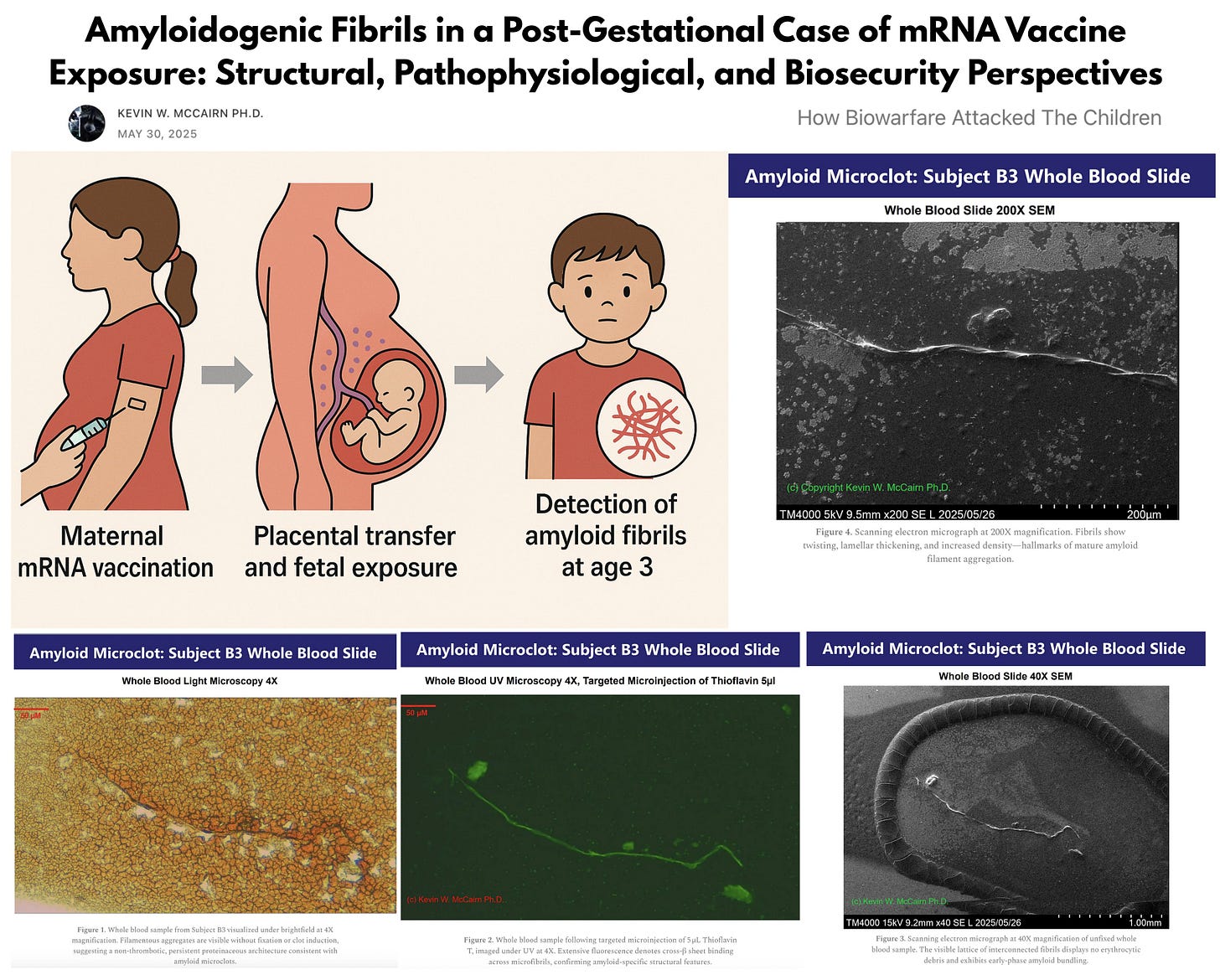
Dr. Kevin McCairn, PhD, a systems neuroscientist with over 25 years of experience specializing in corticobasal ganglia disorders and neurodegenerative disease modeling, joined us to present his sentinel report—the first-ever documented case of prion-like amyloid fibrils in the blood of a chronically ill 3-year-old exposed in utero to Pfizer’s mRNA injection.
Born without vital signs just one week after the mother’s second dose of BNT162b2 (Pfizer), the child required emergency resuscitation. Now experiencing persistent health issues, the child’s blood sample revealed highly abnormal, autofluorescent fibrillar aggregates—confirmed via microscopy and Thioflavin T staining, a selective amyloid-binding dye.
Dr. McCairn’s analysis indicates the presence of misfolded, amyloidogenic fibrin—structures associated with impaired clot breakdown and systemic inflammation. He emphasized that this pathology, linked to spike protein exposure, demands immediate investigation, especially given the forced maternal uptake of genetic injections during pregnancy and the potential long-term risks for an entire generation.
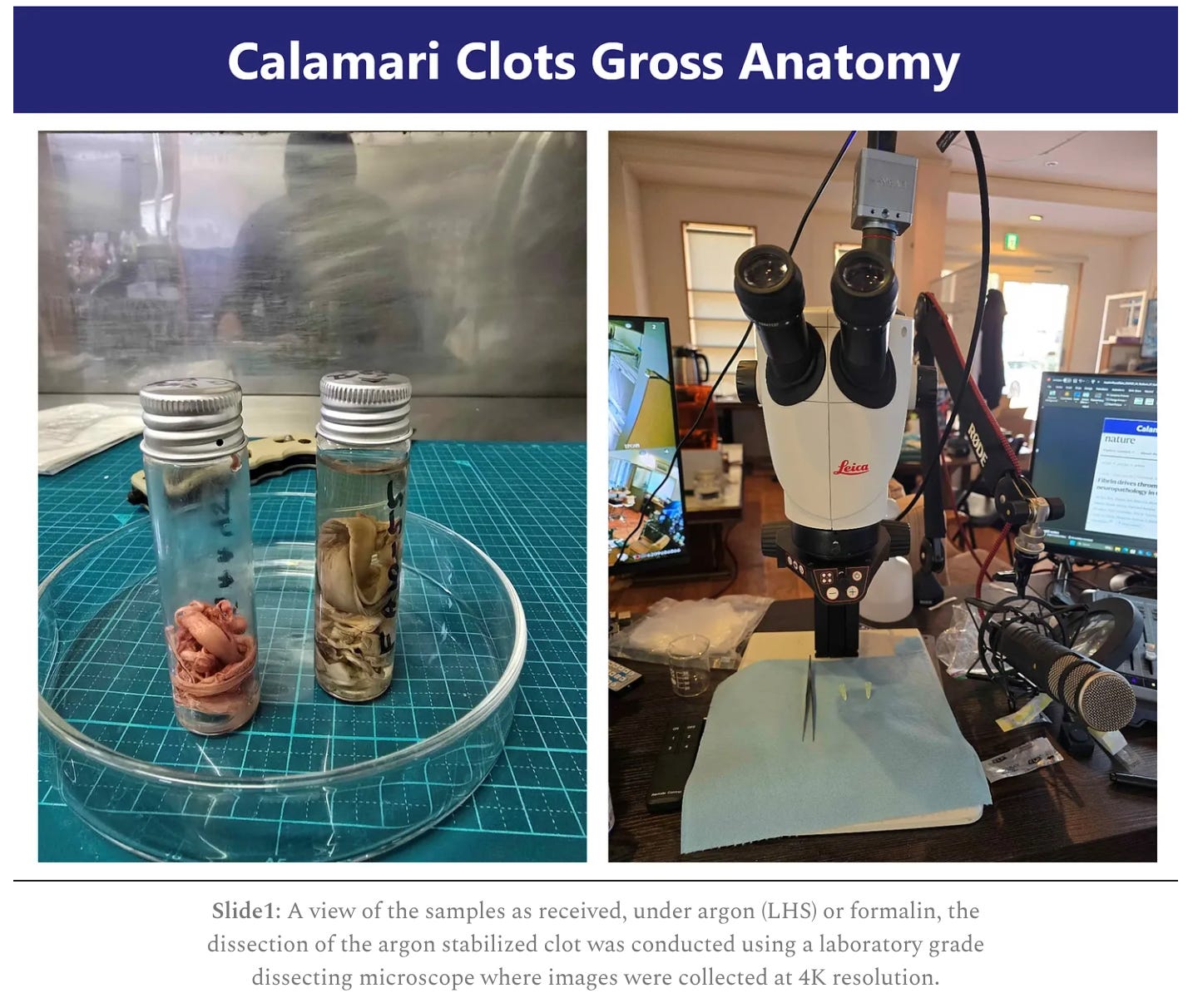
This discovery builds on Dr. McCairn’s prior investigations into large white fibrous clots found in post-mortem human vessels—structures he and his team, including Kevin McKernan, PhD, Shojiro Kato, MD, and Charles Rixey (ret. USMC-CBRN), previously identified as amyloidogenic fibrin using microscopy, Thioflavin T staining, and spectroscopy. These clots showed hallmark features of misfolded protein aggregates, including autofluorescence, twisted fibrils, and beta-sheet structures. Critically, RT-QuIC testing demonstrated prion-like seeding activity, raising concerns that these structures may self-propagate and contribute to systemic disease.
According to Retired U.S. Air Force Major Thomas Haviland’s most recent global embalmer survey, 83% of embalmers reported seeing these large, white, fibrous clots in corpses during 2024.
Below is the full version of Dr. McCairn’s sentinel report, originally published on his Substack.
Amyloidogenic Fibrils in a Post-Gestational Case of mRNA Vaccine Exposure: Structural, Pathophysiological, and Biosecurity Perspectives
How Biowarfare Attacked The Children
Abstract: We report the presence of amyloidogenic fibrils in the peripheral blood of a three-year-old child with documented in-utero exposure to maternal mRNA-based SARS-CoV-2 vaccination. Using fluorescence microscopy and scanning electron microscopy (SEM), persistent fibrillar structures were identified that display amyloid-like morphology and autofluorescent characteristics. This article examines the pathophysiological plausibility of these findings, evaluates counterarguments concerning vaccine component persistence and placental transfer, and contrasts observed structures with canonical amyloids and atherosclerotic plaques. We conclude with a contextual analysis linking these observations to the origins of SARS-CoV-2 and its countermeasures, which emerged from biowarfare-linked research conducted within civilian academic institutions under the rubric of Dual Use Research of Concern (DURC).
1. Introduction: Persistent coagulopathic and neuroinflammatory syndromes following SARS-CoV-2 infection or vaccination have raised concerns about the prolonged biological activity of spike protein. Emerging concerns extend to fetal and early childhood exposure through maternal vaccination. We present a case of amyloidogenic fibril formation in a child three years postpartum following in-utero exposure to spike protein encoded by mRNA vaccination.
2. Case Background: The child was born prematurely at 35 weeks gestation, one week after the mother’s second dose of the BNT162b2 (Pfizer) mRNA vaccine. The child was delivered without vital signs and required emergency resuscitation. The severe temporal correlation suggests a significant adverse maternal-fetal reaction.
Over the subsequent three years, the child experienced recurrent immune dysfunction, including tonsillectomy and multiple surgeries for persistent middle ear infections. An unusual frequency of common infectious illnesses and impaired immune responses prompted further investigation into systemic pathologies.
Whole blood samples were analyzed using fluorescence microscopy with Thioflavin T (ThT) staining and SEM. In both imaging modalities, fibrillar structures with amyloid characteristics were identified.
3. Fluorescence and SEM Imaging: A detailed fluorescence microscopy report reveals widespread ThT-positive fibrillar aggregates in whole blood slide preparations.
SEM imaging corroborated these findings, showing non-denatured, fibrils consistent with amyloid morphology and distinct from canonical fibrin. These structures were present in fresh, unfixed samples, excluding post-mortem artifact.

\



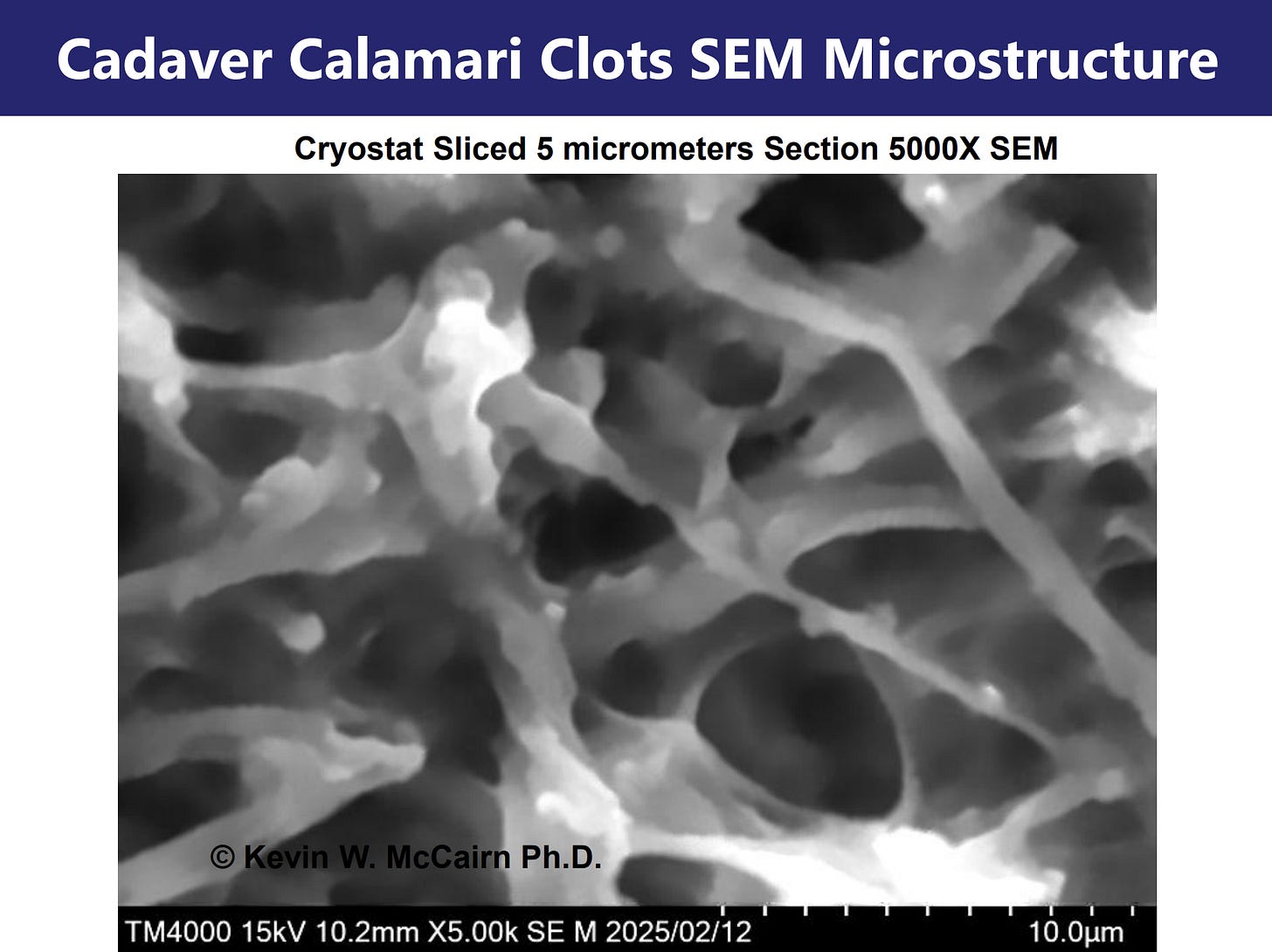
This preservation of twisting amyloidogenic forms across several orders of magnitude in magnification, suggests a consistent and robust self-assembly pattern. Such structural invariance supports the notion that fibrillogenesis follows an energy-minimizing and spatially coherent misfolding pathway. The implication is that systemic amyloidogenic formation is not a localized or transient artifact, but a process rooted in fundamental physicochemical dynamics of protein folding failure.
These comparisons between nano- and macro-structures highlight the conserved geometry of pathological amyloidogenesis. This scale-invariant preservation of fibrillar architecture aligns with prior biophysical studies demonstrating that amyloid formation follows universal thermodynamic pathways, forming twisted ribbon-like or lamellar structures irrespective of protein species or environmental origin (Chiti & Dobson, 2017; Eisenberg & Sawaya, 2017). The similarity across scales—from nanometer-thick fibrils to centimeter-scale clots—suggests a deeply encoded biophysical template likely seeded by persistent amyloidogenic peptides, such as SARS-CoV-2 spike protein.
4. Steelman Rebuttal: Arguments Against Causation and Their Failures
a) Persistence of Spike Protein
It has been widely claimed that spike protein and mRNA clear rapidly from both maternal and fetal systems. However, this assertion is not supported by accumulating empirical data:
The Yale Post-Vaccine Syndrome study (2025) identified circulating spike protein in symptomatic individuals up to 700 days post-vaccination.
Yonker et al. (2023) detected spike protein in serum and exosomes of adolescents weeks to months post-injection.
These findings call into question the original pharmacokinetic modeling used during vaccine approval phases.
b) Transplacental Transfer of LNP and mRNA
The claim that the placenta effectively blocks vaccine components has been refuted:
Swingle et al. (2023) demonstrated delivery of mRNA to the placenta in vivo using ionizable lipid nanoparticles.
Safford et al. (2024) showed that elasticity-tuned LNPs enhanced biodistribution to the maternal-fetal interface.
These findings provide direct evidence that mRNA vaccine components are capable of reaching the fetal environment.
c) Fibril Persistence Without Ongoing Seeding
It is often argued that pathological fibrils require continual reseeding to persist. However, this view ignores well-characterized prion-like behaviors:
Knowles et al. (2014) established that amyloid fibrils can autocatalyze, maintaining themselves once seeded under permissive conditions.
Inflammatory cytokines, cellular stress, and exposure to amyloidogenic peptides can perpetuate misfolding cascades long after the original exposure has ended.
Moreover, while postnatal exposure to SARS-CoV-2 or environmental spike protein cannot be ruled out, the severity and immediate timing of the perinatal crisis—occurring within one week of the maternal injection—strongly implicates the vaccine-induced spike as a precipitating cause. Given the known amyloidogenic motifs of spike protein and the known property of the gene-transfection strategy to restrict biodistribution, the precautionary principle must presume that subsequent exposure to environmental spike would serve only to exacerbate an already-seeded amyloidogenic cascade, not initiate it.
Taken together, these rebuttals demonstrate the scientific basis for sustained fibrillogenesis post-in utero exposure to spike protein delivered via gene-based vaccination platforms.
5. Differential Morphology vs Atherosclerotic and Coagulative Clots
Compared to canonical fibrin-rich clots or atherosclerotic plaques, the observed fibrils differ in both structure and spatial context. These amyloidogenic forms:
Lack lipid or cholesterol cores typically found in atheromas;
Exhibit organized cross-β sheet architecture confirmed via Thioflavin T staining;
Were found circulating freely in whole blood preparations, not embedded in endothelium or vessel walls;
Persist in morphology and fluorescence without the need for clot induction.
The absence of erythrocyte entrapment, the lack of post-thrombotic morphology, and the spontaneous presence of these structures in minimally processed blood underscore a pathological process distinct from ordinary coagulation or arteriosclerotic degeneration. These findings further support the hypothesis that amyloidogenic fibrin can arise systemically and persist independently of classical thrombotic mechanisms.
6. Biosecurity Implications and Structural Novelty
The presence of stable amyloidogenic structures in a child three years post in-utero exposure demands reevaluation of the safety assumptions surrounding mRNA-based interventions. The spike protein has been documented to form amyloidogenic epitopes, and its capacity for proteolytic resistance mirrors that of prions.
Recent findings by Hammerstom and Nyström (2021) have further deepened the concern. Their structural bioinformatics work identified never-before-seen amyloidogenic domains within the SARS-CoV-2 spike protein, which include attack surfaces aligned with known neurodegenerative and inflammatory mechanisms. These motifs—conserved in both the virus and the encoded vaccine spike—were propagated globally through a mass public health measure based on gene-transfection technology.
Given that over 13 billion doses of mRNA-based COVID-19 vaccines have been administered worldwide (WHO COVID-19 Dashboard), and that global SARS-CoV-2 infection numbers surpass 770 million individuals, a substantial portion of the human population has been exposed either to the pathogen or the transfected antigen. Furthermore, the continued inclusion of mRNA vaccination in pediatric and booster schedules amplifies this exposure.
This raises alarming questions about the cumulative biological burden of such widespread contact with a protein that may harbor intrinsic misfolding and aggregation potential. The possibility that environmental spike exposure serves as a reinforcing mechanism for pre-seeded pathology—especially in developmentally primed systems—necessitates urgent scrutiny under the precautionary principle and renewed international bioethics standards.
7. Conclusion
This case represents a sentinel event that underscores the need for systematic, high-resolution evaluation of post-vaccine biology—especially in pediatric and developmental contexts that have the shadow of events being precipitated by biowarfare research. The persistence of amyloidogenic fibrils years after gestational exposure cannot be dismissed by outdated pharmacokinetic assumptions. Only rigorous post-market surveillance, independent replication, and transparent access to proprietary vaccine data can resolve these urgent questions, especially and until the threat from synthetic, biowarfare spawned amyloids/PRIONS has been contained.
8. Bibliography
McCairn, K. W. et al. (2024). Cadaver Calamari: Amyloidogenic Fibrin Structures in Post-Mortem Human Vessels. Substack. https://kevinwmccairnphd282302.substack.com/p/cadaver-calamari-amyloidogenic-fibrin
Chiti, F., & Dobson, C. M. (2017). Protein misfolding, amyloid formation, and human disease. Annual Review of Biochemistry, 86, 27–68. https://doi.org/10.1146/annurev-biochem-061516-045115
Eisenberg, D., & Sawaya, M. R. (2017). Structural studies of amyloid proteins. Annual Review of Biochemistry. https://www.annualreviews.org/doi/10.1146/annurev-biochem-061516-045104
Swingle, B. et al. (2023). Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta. J Am Chem Soc, 145(8), 4691–4706. https://doi.org/10.1021/jacs.2c12893
Safford, H. et al. (2024). Probing LNP Elasticity on mRNA Delivery to the Placenta. Nano Letters, 25(12), 4800–4808.
Yonker, S. et al. (2023). Circulating spike protein in post-mRNA vaccine myocarditis. Circulation. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.061025
Patterson, B. et al. (2024). Persistence of spike protein and immune dysfunction. Yale Immunology Report. https://news.yale.edu/2024/02/19/immune-markers-post-vaccination-syndrome-indicate-future-research-directions
Knowles, T. P. et al. (2014). The amyloid state and its diseases. Nature Reviews Molecular Cell Biology. https://www.nature.com/articles/nrm3810
Hammerstom, L., & Nyström, S. (2021). Amyloidogenesis of SARS-CoV-2 Spike Protein. Journal of the American Chemical Society. https://pubs.acs.org/doi/10.1021/jacs.2c03925
WHO COVID-19 Dashboard. (2024). Global infection and vaccination statistics. https://covid19.who.int/
Dr. Kevin McCairn currently offers testing of blood samples to assess amyloidogenic burden. For details on how to submit a sample, visit: https://synapteklabs.com/protocol-on-sending-blood-samples-2/
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.